Updated | Hundreds of spit samples have been traveling through the mail across the U.S.-Canadian border. Why? The samples are part of a study focused on genetic testing for breast cancer. Saliva is needed for that—and so are Canadians.
Regulations in the U.S. make it more difficult to conduct a study in which participants receive genetic test results without involving their doctors. Canada is less strict about how genetic test results are released.
Testing for BRCA, a gene that mutates in such a way that increases the risk of breast and ovarian cancers, has been available for many years. But the diagnostic has been limited to women with a family history of these diseases in Canada—or to those with the money and a willing doctor to help them order it in the United States. A U.S. company, Veritas Genetics, believes that expanding genetic testing for two BRCA mutations would enable women who would not otherwise be screened to catch their cancer early.
The company is conducting this study with Dr. Steven Narod at Women's College Hospital in Toronto, Ontario. Narod was one of a group of scientists to identify the BRCA genes and its links to breast and ovarian cancer risk.
So far, half a dozen people out of the nearly 200 who have participated have tested positive for the BRCA gene. A few of those people may not have otherwise been considered eligible for screening, according to Veritas Genetics. (The tests are being sold for $165, or about 200 Canadian dollars.)
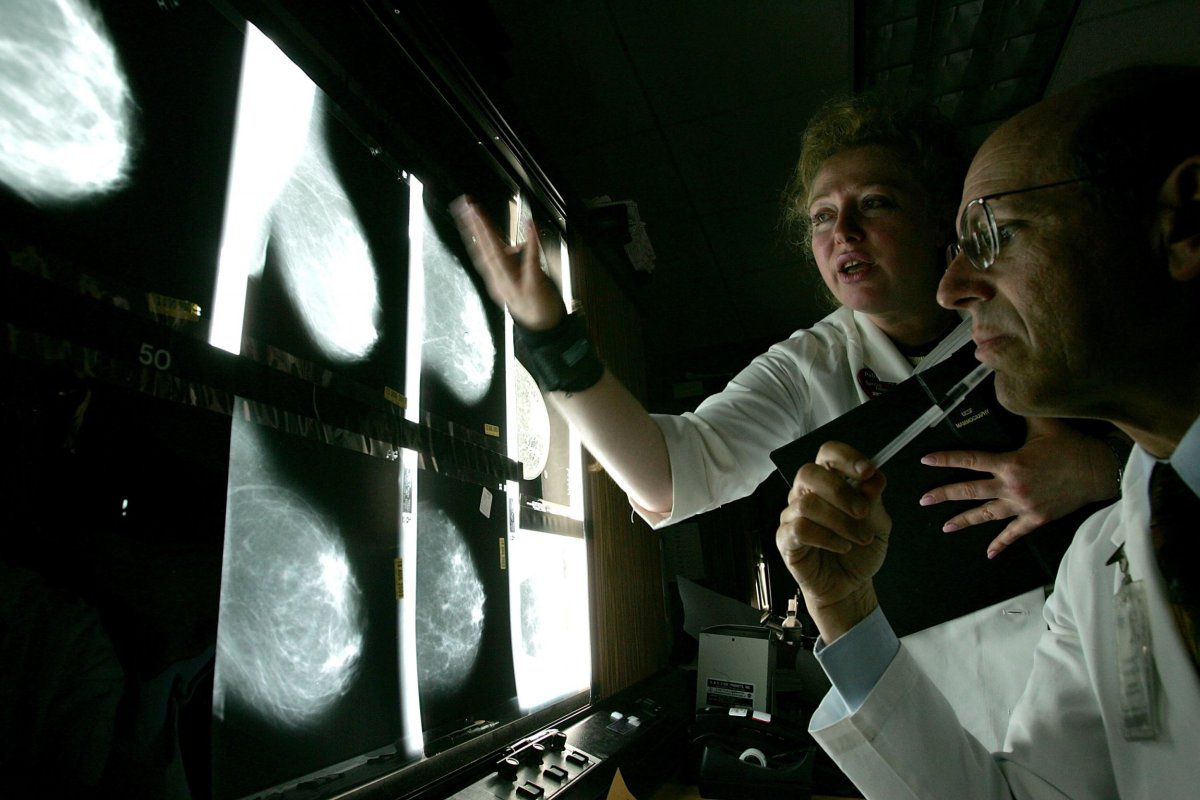
"We're most interested in answering this question: if we offer this test to the general population, how likely is it that people are going to take us up on it, how likely are we to find a mutation, and can we measure the health benefit to the individuals and the population based on finding those individuals," he said.
Narod's work may also help solve a problem at the heart of BRCA testing: if detecting certain BRCA mutations can be so important to a person's health, can the tests be implemented widely in an effective way? How could that be done?
"To some degree, the devil is in the details," said Dr. Matthew Yurgelun, an oncologist at the Dana-Farber Cancer Institute. "You can say that this should be done, but the actual implementation of it is different than just saying that the benefits are there to begin with." Measuring the rate at which people who carry the mutation go for follow-up care is one lingering question, especially in the United States where universal healthcare coverage is still an unrealized dream. Making sure that people understand the potential for false negatives would also be important.
"You can say something has indisputable benefits and should be done, but you can also say we don't know how to do it," Yurgelun said.
Narod is following-up with people who participated to see how knowing their mutation risk might impact their health and decisions. However, he doesn't expect that it will be a representative sample of the population.

Direct-to-consumer genetic tests are in a regulatory bind in the United States. Until recently, the FDA said they would not allow companies to sell genetic tests directly to consumers if those tests provide health information, like the risk a person might have for a disease. In April, the agency authorized 23andMe to start selling tests to provide genetic risk information about 10 diseases. Breast cancer and the BRCA gene were not on the list of authorized conditions.
Until the FDA authorizes companies like Veritas to sell BRCA tests directly to consumers in the United States, they must have a doctor's note before they can run the test. Veritas also requires physician approval for all clients, including those who do not live in the United States.
However, Canada has a different regulatory framework for direct-to-consumer tests—and, of course, a different insurance framework. BRCA tests are free through the universal health plans provided by provinces, but only for certain people. Factors that qualify someone for a free test can vary between provinces, but most require that a living member of a person's immediate family be diagnosed with breast or ovarian cancer and have tested positive for the gene. In some cases, members of a group known to be at elevated risk are also eligible.
Unfortunately, there can be wait times for people on both sides of the border to see a genetic counselor. But since the Veritas project has stayed relatively small so far, Narod said there is no reason to believe people screened will overwhelm the hospital's existing resources.
Whether Veritas's initiative is just the beginning of a new era in BRCA screening is still an open question, but Narod is optimistic. "I think this is the way the future is going to go."
Correction: An earlier version of this story mistakenly said that doctors do not need to approve genetic test results in Canada. Veritas requires that for any test, including the BRCA tests sent to Canada.
Uncommon Knowledge
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
About the writer
Kate Sheridan is a science writer. She's previously written for STAT, Hakai Magazine, the Montreal Gazette, and other digital and ... Read more
To read how Newsweek uses AI as a newsroom tool, Click here.