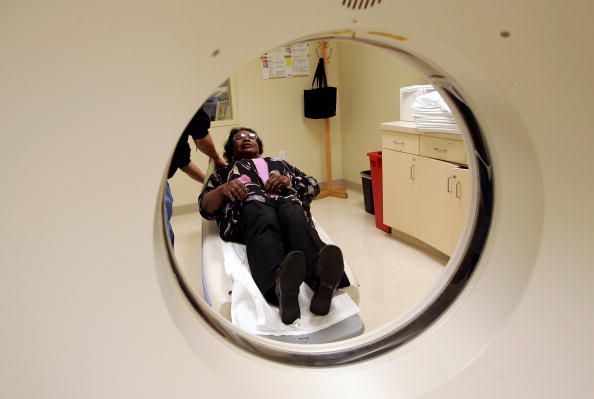
Finding the right clinical trial can be hard for cancer patients. So can the experience of participating in one. Sometimes, it's "almost as bad as dealing with Comcast or the worst airline," Paul Wicks said in an interview for Newsweek's Cancer Issue. He's the vice president of innovation for PatientsLikeMe, a personalized health network and research company that has partnered with pharmaceutical companies, academic institutions and nonprofits. He explained that some patients have such awful experiences in clinical trials that they drop out or actively dissuade others from participating. That's obviously not good for patients or for researchers. In a follow-up interview, Wicks spoke to Newsweek specifically about how clinical trials—from recruitment to the study itself to the aftermath—could be better designed, keeping the patient experience in mind. Edited excerpts follow.
You've told me that patients who do find one often have bad experiences. Why is that?
The process of being in a clinical trial is very confusing initially to people. They will be bombarded with lots of very technical information. They may have to look at informed consent documents that could be 30, 40, 50 pages long, full of very complicated-sounding medical terms and jargon, with only limited time to have that explained to them by their clinician. Then if they do decide to enroll and take part, what tends to happen is there's a large burden on them to come to the study site for follow up visits and these can be very time consuming. Many hospitals are in places that are hard to reach, in big cities. Not enough parking. Public transport can be difficult for people living with medical conditions. These are all common issues. It's just a big ask of people to commit their time to research.
What's wrong with the philosophy of how many clinical trials are run?
If I were to be blunt I would say that they are designed almost entirely with the scientific objectives in mind and with relatively little thought into how the participants could fit this study into their lives. So at their worst, a trial is taking the approach that you might take with guinea pigs. You know, we have them in cages and we can control all the variables and we can get a lot of data out of them and we can do our research projects. But we don't necessarily prioritize the guinea pigs' engagement and participation and you know, sense of holistic wellbeing the way we need to with people. That shows itself in arduous protocol—they're very burdensome—minimal thanks for patients, a lack of feedback, a lack of attempting to teach people and educate people while they're in trials so they can learn whilst they're in the study, which is why a lot of people take part. And that's really disappointing. I think we're failing to complete the social contract that's the reason a lot of people volunteer to take part in research in the first place.
What is that social contract?
Perhaps a way that we could ask an individual, "Why are you taking part in this study?" And I think a lot of people are conditioned to say, "Oh it's altruism, it's for the benefit of other people." But I think that we should encourage people to admit that, actually, they want to get a better outcome for themselves, or they want to learn more about their condition, or they want to spend more time with their doctor. They may find out that actually the odds of you being helped personally are low, or that actually you're not going to see your doctor or that you're actually not going to hear about your results—that there will be no results for many years and no one is going to give you any personalized advice or feedback. Sometimes it's about expectation setting.
I don't think doctors sit around all day thinking how do I create a dehumanizing process for people to go through that will be a pain. They're trying to extend people's lives and get drugs out there that work. I think in their singular focus on that—and the lack of resources available to them and the lack of guidance, by the way—they'd have to make this stuff up. And they wouldn't exactly be rewarded for it by their institutions or by the people that pay for this research. They end up trapped by that same system. There needs to be a minimum standard.
How do you measure people's experiences on a larger scale and what kind of impact do bad experiences have?
People might have heard of the net promoter score. Anytime they've filled in a questionnaire at a restaurant or a hotel or something like that, and it says, "How likely are you to recommend this service to a friend or family member?" So we asked that question to people who've been in clinical trials. What we found was that on average, the experience of people who've been in trials was very poor. There were more detractors or people who were sort of passive about the whole experience than people who were positive.
The way that you would energize the community is if you had a lot of people who took part in a trial being really positive about it and you know evangelizing to their friends or peers with the same condition to say, "Wow you should take part in this clinical trial. I'm learning so much about myself. I'm being looked after really nicely. I understand what my role is. I'm being respected. They're really seeking my input on how to do things better." And that way each patient would become like an advertisement for the trial. But what it seems like is happening now is patients are saying, "I'm in a study. It's really hard. They keep me in the dark. They keep changing my appointments. It's really difficult to get there. I don't know if I'm having side effects from this medication. I had to come off this other medication I was stable on before. And I just find the whole thing exhausting. I wouldn't sign up for it if I were you." And you can imagine, if you're the investigator trying to get a sample of a thousand people, how's that going to go in either of those two scenarios?
If you were a brand, if you were a company, if suddenly someone starting saying, "Oh, all the trainers at Crossfit are really horrible and their fees are really expensive." You know what that kind of negative marketing would do.
What concrete steps could trial designers take to make the search and recruitment, the trial experience and the aftermath better for patients?
Basically the answer to all three of your things is partner with patients and have patients, and caregivers potentially, empaneled on the steering committee of the trial right from the get go. Patient advocates should be paid for their time. They should be supported to attend the meetings. They should be trained. And they should have just as many rights as anybody else there to you know make a suggestion or to vote something down. But equally responsibility to take on a body of work. They could perhaps go and use other patients they know in the community as a sounding board for different ideas or to help develop some of the writing.
That's the most obvious concrete thing. And the reason I say that is that from all that I've seen here, I can't tell you what it's like to have bladder cancer because I don't have it. And I can't tell you what it's like to want to do a study on bladder cancer in 2017. It would be sort of patronizing of me as a professional to say, "This is the solution." But what I should be able to do is open the door for patients like that to get in and then they can say from their perspective what is the right approach. When it's normal to bring a patient in early on I think we'll see significant improvement in all the issues that we're experiencing now.
Tell me more about what could help while a clinical trial is running?
Right now there's not really a systematic way for trials that are already running to gather feedback from their participants. So I think even something as simple as a number you could text with questions or comments or complaints would be helpful. I think you could be more proactive than that and after each study visit ask patients for feedback. Like a customer satisfaction survey after every visit. That seems wildly easy.
It's not difficult to do this but you have to want to hear it. If they're all saying, "You made me wait naked in a paper gown for an hour on a table and I felt embarrassed," then you need to do something to change that. They might say, "I don't think you treat me with respect." They might say, "I'm bored." I think patients are suddenly meant to get this halo and be all-tolerant saint-like individuals. But yeah it's boring sometimes, sitting in a scanner for hours and hours, or getting chemo for hours and hours, or what have you. If in your mind you have a limited time left on this Earth, how much of it do you want to spend in a clinical trial dealing with the trappings of that? We cannot just assume that people will go through anything we throw at them.
What about the aftermath?
I think there needs to be a little bit of soul searching about how we give the results back to our study participants. Most studies are not open access, so most people that took part in a clinical trial and wanted to read the final study publication, generally speaking would have to pay $35 for the privilege for renting that paper for 24 hours. Which seems like a bit of a slap in the face. The study investigators could make that paper free for a one-off payment of about $3,000, and they often don't, citing budgetary constraints. Some of these things I've said—involving patients from the start, that requires cultural change and overcoming inertia and that will be hard, there'll be some process. Cutting a check for three grand for a permanent resource that the world can access for free, seems like a no brainer.
How would improving patient experience improve research?
The term that everybody uses in industry is return on investment. There's been a question of, "If I do all this work what do I get back?" So I think the first thing to say is maybe we shouldn't be so hasty to figure out what the ROI is when we just haven't been doing it at all for 50 years. Maybe we need to do it because it's a good thing to do and then we'll figure out the benefits later.
I think the most obvious benefit would be an increase in speed of enrollment, and that means the study completes faster. You would reduce dropouts and I think you would improve statistical power, which means an increase in our confidence to know that we've answered the question conclusively. I think you'd see more adherence to the study protocol so you'd get better data. So really it's better on nearly every metric that matters to a researcher. And then even at the end once the study is complete, if they make sure the research is disseminated to patients and is accessible, more people will read their research, which means more people will benefit from it. It really does feel like it's wins all around.
Is there anything else you want to add?
There's a question you can pose to your readers to say if you had to how would you fit in this study burden into your life? Imagine a two hour drive to the hospital and then five hours in the clinic and then a two hour drive back, if you had to do that every two weeks. What would you have to change about your life and your responsibilities to fit that into your life now when you're healthy? Now imagine it when you're sick. What healthy readers will think is, "Oh well they've got cancer, they should just suck it up. They should just do whatever is needed because it's a fight against cancer." That only gets you so far. If it was that simple recruitment rates wouldn't be terrible like they are.
This is a niche experience. Hopefully most people won't go through this, so it can be hard for them to understand and empathize what is this really like.
Uncommon Knowledge
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
About the writer
Stav is a general assignment staff writer for Newsweek. She received the Newswomen's Club of New York's 2016 Martha Coman Front ... Read more
To read how Newsweek uses AI as a newsroom tool, Click here.