Cholesterol could possibly be linked to increased risks of cognitive decline and dementia.
Deposits of proteins known as tau—associated with the onset of Alzheimer's disease—in the brain are connected to the accumulation of a form of cholesterol known as cholesteryl esters, according to new research in the journal Neuron.
Tangles of tau proteins in the brain drive cognitive decline, as it causes nearby brain tissue to start to degenerate. In the United States, about 5.8 million adults have Alzheimer's disease and other dementias, according to the U.S. Centers for Disease Control and Prevention.
One genetic risk factor for Alzheimer's disease is a gene called APOE, which activates immune cells in the brain that can cause damage to brain tissue if activated in the wrong way or at the wrong time. APOE is also involved in the carrying of cholesterol around the body in the blood.
"APOE transports lipids and cholesterol both in the bloodstream and in the brain. In the brain, APOE is produced by cells called glial cells, both astrocytes and microglia. In and of itself, APOE does not damage the brain," paper co-author David M. Holtzman, a professor of neurology at the Washington University School of Medicine in St. Louis, told Newsweek.
"In Alzheimer's disease and some related disorders called tauopathies, the protein tau, which is an abundant protein in neurons, begins to clump together and build up. When this happens, neurons and their connections begin to die. APOE can modulate the amount of brain damage that occurs when tau accumulates. APOE4 appears to make the neuronal injury due to tau much worse."
The researchers tested the connection between the APOE gene, cholesterol, and brain damage by modifying the gene in mice. These mice already had a high-risk tau gene that makes them accumulate tau rapidly, showing neurodegeneration at six months of age and having severe brain damage by 9.5 months to the point where they can no longer perform basic tasks.
Some of the mice had their APOE genes removed and replaced with human APOE genes—some with APO3, which has an average risk of Alzheimer's, and APOE4, which doubles or even triples the risk. Other mice did not have the gene replaced at all.
"Tau is an abundant normal protein in the brain. It appears to play a normal function in neurons. In Alzheimer's disease and related disorders, it takes on a toxic function when it clumps together and aggregates," Holzman said.
"There are several reasons why it can aggregate. It aggregates to some extent with aging. However, it is strongly driven to aggregate when it carries mutations that cause rare forms of diseases called tauopathies. Another factor that accelerates its aggregation is the other protein that builds up in the brain in Alzheimer's disease called amyloid."
The researchers found that mice that carried APOE4 had distorted brain lipid metabolism, meaning that the same areas of their brain that were damaged by tau were accumulating strange patterns of lipids, including cholesterol and over 180 other types. Immune microglia cells were found to be filled with cholesteryl esters.
"It appears that when certain types of cholesterol and other lipids accumulate in cells in the brain called microglia, this leads to higher levels of inflammation. The inflammation is likely what increases the aggregation and accumulation of tau," Holzman said.
APO3 did not cause this effect on the brain, the researchers found.
To determine if removing the lipids could prevent neurodegeneration in the mice, the researchers used an experimental drug known as an LXR agonist which lowers cell lipid levels. When the APOE4 mice were given the drug, named GW3965, the scientists found that those whose brains would usually show large amounts of damage had a lot more brain volume than the mice who took the placebo drug.
They also had much lower levels of tau, less synapse loss, and less brain inflammation.
"LXR agonist stimulate the production of a number of genes, one of which is called ABAC1. ABCA1 increases the export of lipids from cells which is probably why an LXR agonist decreased the buildup of lipids," Holzman said.
This implies that reducing brain lipid levels may be able to prevent the onset of Alzheimer's and other neurodegenerative diseases.
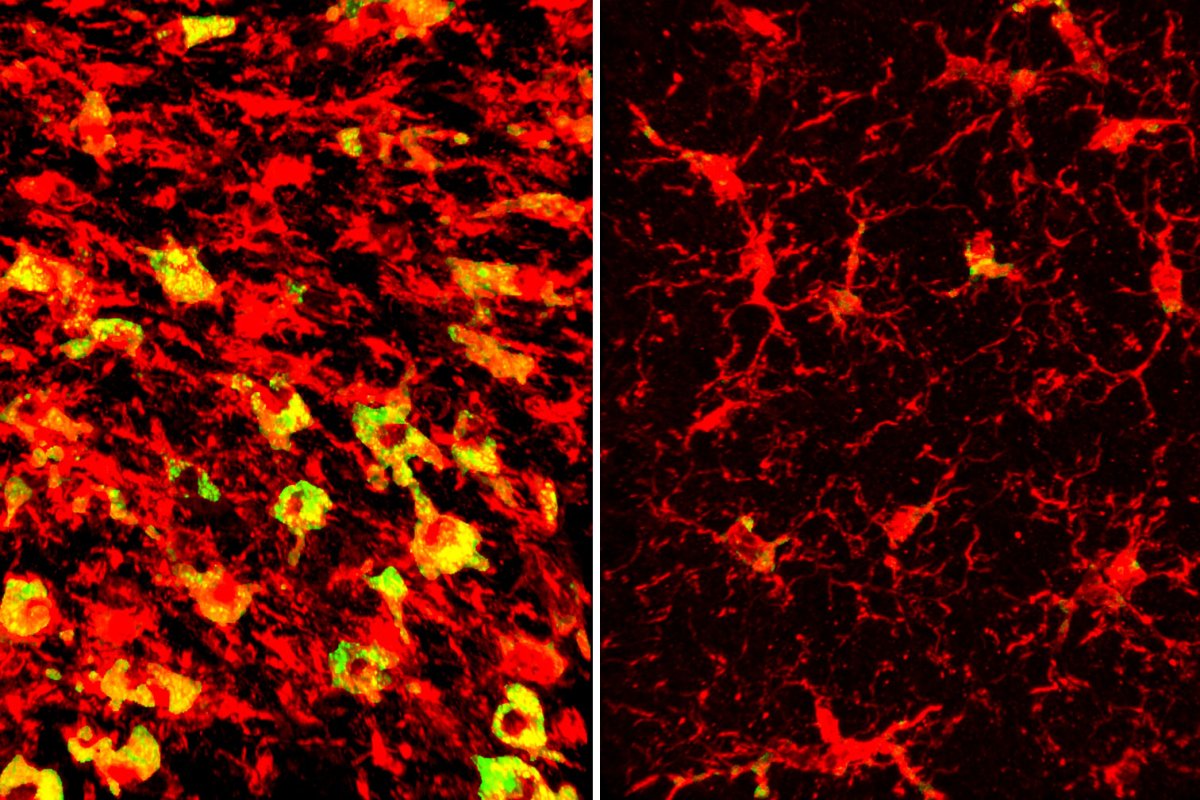
The researchers also found that the LXR agonist drug worked by upregulating a gene known as Abca1, which works by moving lipids including cholesterol out of cells. By cutting out the drug and using genetic methods to boost Abca1's effects, the researchers found the same outcome: less brain lipids, less tau, and better brain volume.
While they hope to develop these findings to apply them to humans, one major obstacle includes the fact that LXR agonists cause fatty liver disease due to their impacts on liver metabolism.
"LXR agonists can also cause an increase in cholesterol synthesis in some cells, especially liver cells. This can cause 'fatty liver.' There are different groups working on developing compounds that have some actions like LXR agonists which increase the export of lipids from inflammatory cells but don't increase cholesterol synthesis in cells like liver cells," Holzmann said.
If this side effect can be removed from the drug, then it could be used not only to treat neurodegeneration but also atherosclerosis caused by cholesterols in the circulatory system.
"There's a lot of similarity between the mechanism that's driving immune cells to damage the brain in Alzheimer's disease and the one that's driving the same kinds of immune cells to cause vascular damage in atherosclerosis," Holtzman said in a statement.
"In both cases, lipids accumulate in immune cells, causing them to become hyperinflammatory and damage nearby tissues. Getting rid of that lipid accumulation may have double benefits for human health."
Update 11/27/23, 11:18 a.m. ET: This article was updated with comment from David M. Holtzman.
Do you have a science story to share with Newsweek? Do you have a question about neurodegenerative conditions? Let us know via science@newsweek.com.
Uncommon Knowledge
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
About the writer
Jess Thomson is a Newsweek Science Reporter based in London UK. Her focus is reporting on science, technology and healthcare. ... Read more





