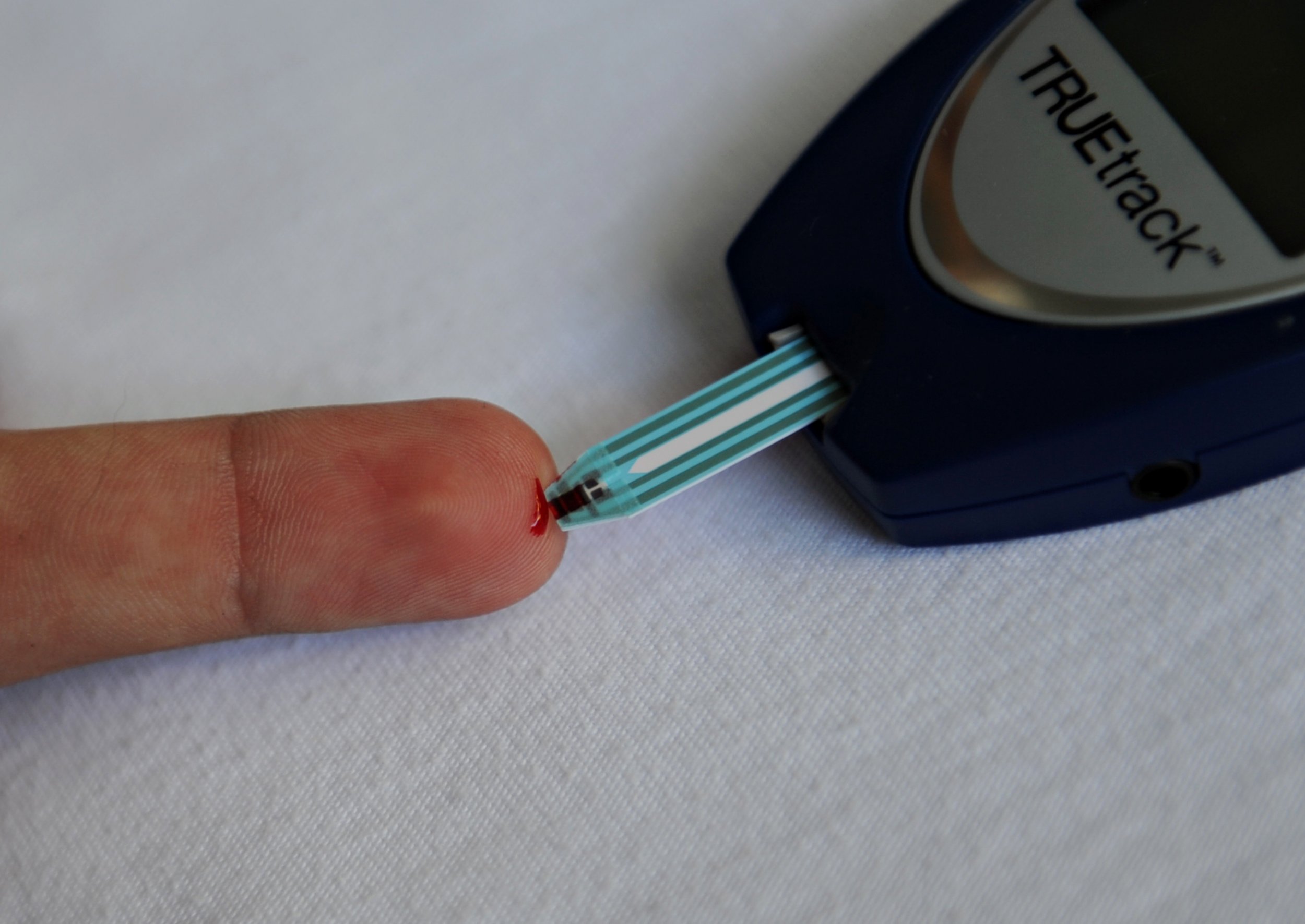
After years of preliminary studies, researchers at the University of Tampere, in Finland, are finally ready to test their diabetes vaccine in clinical trials. Following evidence that the new approach prevented type 1 diabetes in mice, the team is ready to test its prototype on people.
The work focuses on enteroviruses known to cause the disease. Enteroviruses are RNA-based viruses linked to many human ailments, including polio; the common cold; hand, foot and mouth disease; and several others. This group of viruses has been roughly categorized into four types. One of these types, Coxsackie B viruses, includes those responsible for type 1 diabetes.
Led by virologist Heikki Hyöty, the researchers had identified the exact enteroviruses responsible for triggering type 1 diabetes. With that information in hand, they set out to create a prototype vaccine. Just like many other immunizations, the vaccine leads the immune system to recognize the culprit virus and stop it in its tracks. Studies in mice showed the experimental vaccine to be both safe and effective at preventing the disease, giving Hyöty and colleagues a clear sign that human studies were warranted.
The first phase of clinical trials, scheduled to start next year, will focus on a small group of adults in order to verify that the vaccine is safe. If that study pans out, the immunization will be studied in children, again to test its safety. If that second phase delivers positive results, the vaccine will be tested in a larger group to see if it does indeed prevent type 1 diabetes. The entire process, including confirming whether the immunization works, could take up to eight years.
Type 1 diabetes occurs when cells that normally produce insulin in the pancreas stop working. Researchers have been trying to parse the link between enteroviruses and type 1 diabetes for decades. Studies have honed in on a particular group of viruses that directly infect those insulin-producing cells. Finland, where this vaccine candidate was developed, has an unusually high number of type 1 diabetes cases. This variety of diabetes is far less common than type 2 diabetes and the vaccine would not work for the latter disease.
Because enteroviruses are implicated in so many diseases, the researchers are optimistic that the prototype vaccine could lead to others for additional illnesses, including the common cold.
"The aim is to develop a vaccine that could prevent a significant number of type 1 diabetes cases. Additionally, the vaccine would protect from infections caused by enteroviruses such as the common cold, myocarditis, meningitis and ear infections. However, in light of current research, the vaccine could not be used to cure existing diabetes," said Hyöty in a statement.
Uncommon Knowledge
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
About the writer
Jessica Wapner is the science editor for Newsweek. She works with a talented team of journalists who tackle the full spectrum ... Read more
To read how Newsweek uses AI as a newsroom tool, Click here.