This article was originally published on The Conversation. Read the original article.
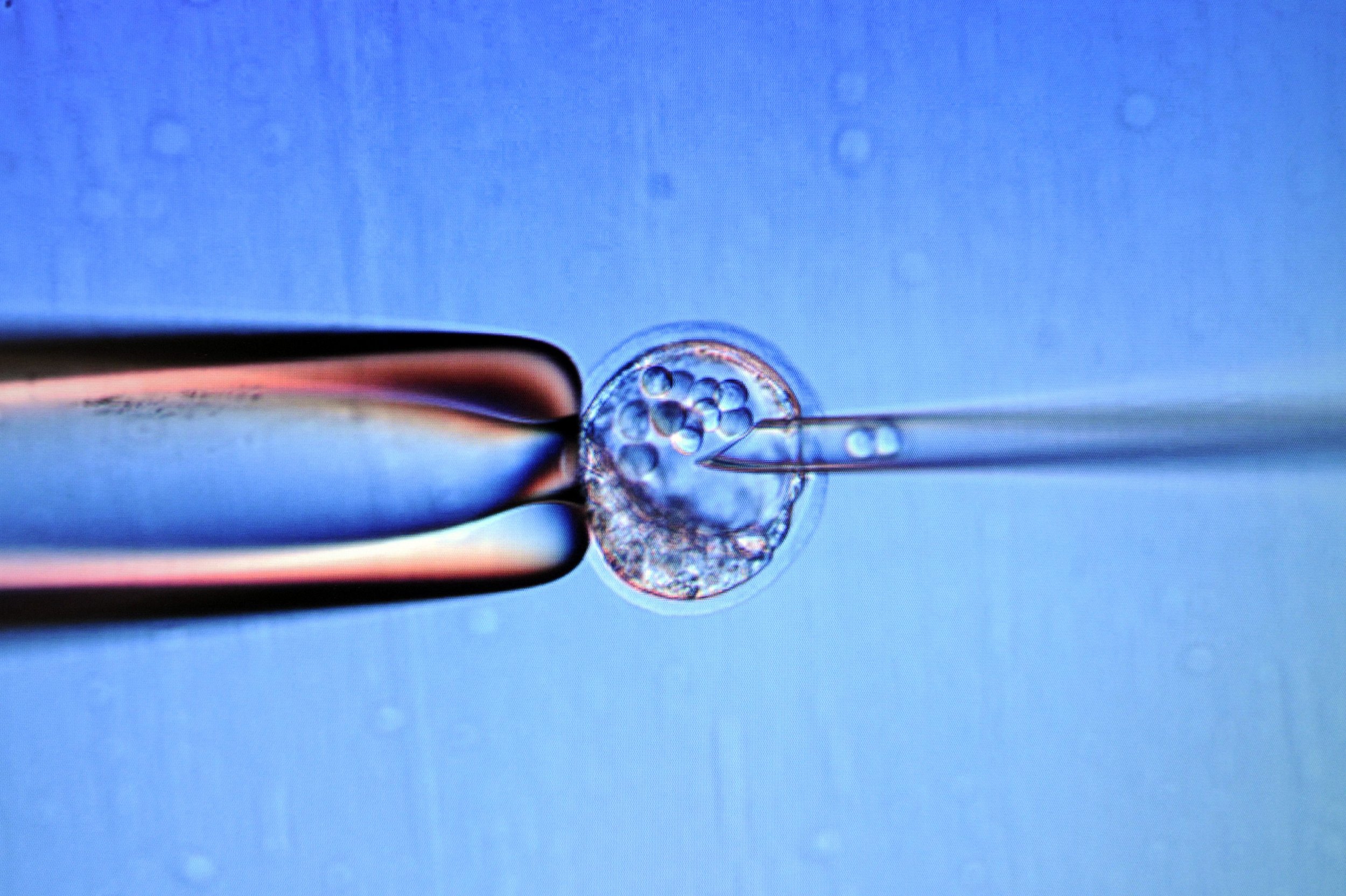
Scientists have for the first time shown that fully mature egg cells can be grown in the lab, raising hope for new infertility treatments.
Until now, researchers have only been able to produce cells that resemble sperm or eggs, but which can rarely produce live offspring because of abnormal organisation of their genetic material. But a team at Kyushu University, Japan, has now turned stem cells from mice into mature eggs than can be fertilized and develop into healthy, fertile adults. This could lead to a way for women who can't naturally produce working eggs to have new ones made from their own cells.
Embryonic stem cells are living cells taken from an embryo that have the ability to develop into any other kind of cell. The researchers from Kyushu University team previously demonstrated that, under the right conditions, these cells could be turned into primordial germ cells, immature embryonic versions of sperm and eggs. But because they are immature, these germ cells can't produce any offspring.
So the researchers adapted their methods to encase the stem cells in other cells taken from a mouse's foetal gonad (the developing ovary or testis). This recreated an environment more like an ovary and, over a period of four to five weeks, the team saw the stem cells develop into cells resembling mature eggs.
Functioning egg cells
While the cells looked like mature eggs, the key question was whether they actually were functional egg cells. The team compared their lab-grown eggs with ones from an ovary and found they were the same size and organized their genetic material in similar patterns.
The researchers also showed that their eggs could be fertilized, implanted into a surrogate female and go on to produce live offspring. But only a very small number of their embryos created in this way developed fully to term—just 3.5 percent of all the embryos they transferred. Importantly though, the team reported that "all the obtained pups grew up normally without evidence of premature death."
As all good scientists should, the researchers then replicated their experiments to test how robust their technique was. Initially, they used embryonic stem cells in their experiments, but these create an ethical dilemma because an embryo has to be destroyed to produce them.
In 2006, however, another researcher named Shinya Yamanaka and his team found that turning on just four specific genes in normal adult cells gives them all the potential to develop into other cells just like embryonic stem cells, but without the need to destroy a single embryo. The latest research showed that eggs made from these "induced pluripotent stem cells", or IPSCs, were just as capable of being fertilized and producing healthy adult offspring as embryonic stem cells.
Research challenges
The findings from this study have clear implications for the treatment of human infertility. Being able to manufacture working eggs from regular cells could allow doctors to provide an alternative for women who don't naturally produce functional eggs. But, as with all research studies like this, there are still some limitations that need to be addressed.
First, the overall success rate of this technique is still low—just 3.5 percent of all embryos implanted gave rise to live offspring, compared to 30 percent of those currently used for human IVF treatment. Obviously, this would need to be improved, potentially by using different lab conditions, hormonal treatments or by encasing the stem cells in adult gonadal cells rather than foetal ones. However, improving the efficiency of such complex lab techniques can be very difficult.
Second, this study was conducted in mice and not humans. While the two species are similar in the way their eggs and embryos develop, there are some key differences. So scientists still need to prove they can replicate the technique with human cells.
Finally, while the researchers went to great lengths to show that the eggs, embryos and offspring generated in this study were "normal", the lab-grown eggs did display altered genetic patterns and unusual placenta growth. This means we need to research the full impact of the techniques used in this study on the long-term health of any offspring generated.
Still, the findings from this study open up new possibilities for the preservation and even restoration of fertility in women. As always these kinds of scientific breakthroughs, while there are clear benefits for many people, they also carry potential ethical implications. But the team at Kyushu University have pushed the boundaries of reproductive biology, opening new avenues that may one day help millions.
Adam Watkins is a research fellow, Cell & Tissue Biomedicine, Aston University.
Uncommon Knowledge
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
About the writer
To read how Newsweek uses AI as a newsroom tool, Click here.








