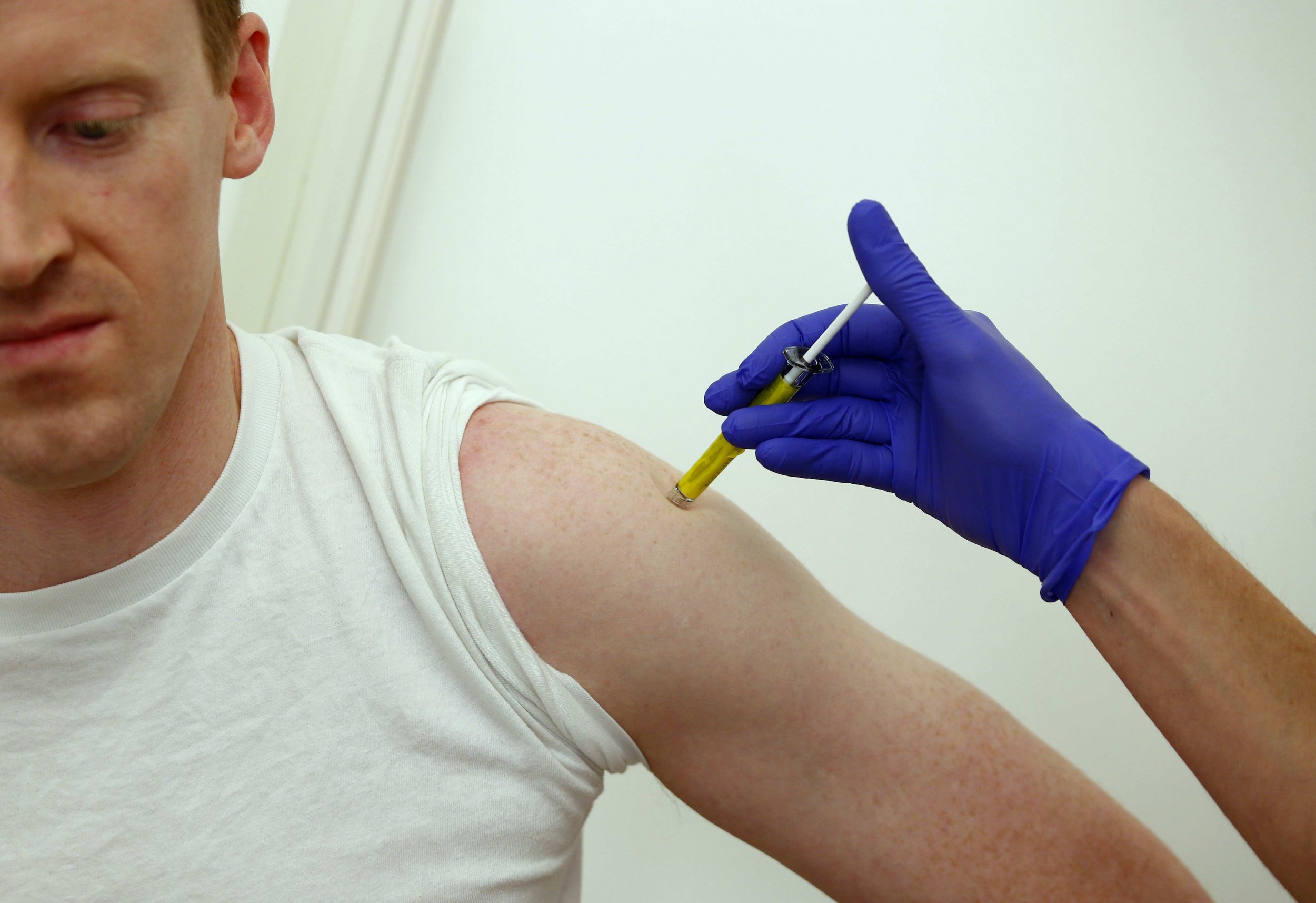
The first doses of an experimental Ebola vaccine are expected to arrive in Liberia on Friday.
Calling it a "major milestone," GlaxoSmithKline, the pharmaceutical company behind the vaccine, said in a statement the first large-scale trials in the coming weeks could involve up to 30,000 volunteers, including health-care workers. The shipment contains 300 vials of the vaccine.
More than 8,675 people have died and 21,797 have been infected with Ebola in Liberia, Sierra Leone and Guinea, the three-worst hit countries, according to the World Health Organization (WHO). Sierra Leone has seen the highest number of cases, 10,340, and Liberia has seen the most deaths, 3,605.
Front-line health-care workers have been at particular risk during the epidemic: Of the 828 who have been infected, 499 have died, according to WHO. In December, the U.S. opened a 25-bed hospital in Monrovia, Liberia, especially to treat health-care workers.
Ebola cases have been declining in recent weeks, marking a turning point in the fight against the deadly disease, the Guardian reports. Mali, which saw eight cases and six deaths from Ebola, was declared free of the Ebola transmission earlier this week. Liberia reported eight Ebola cases each of the past two weeks, down from a peak of more than 300 per week in August and September. Guinea saw case numbers declining for the third week in a row and, while still high, Sierra Leone saw 117 cases last week, compared to 184 the week before, according to WHO.
The GSK vaccine is currently in phase I trials in the U.S., the U.K., Canada, Switzerland and Mali. The vaccine has an acceptable safety profile, according to scientists, and will now move into phase III trials in West Africa, led by the U.S. National Institutes of Health (NIH). If standards are met, 10,000 people will receive the candidate Ebola vaccine and 10,000 will be given a placebo vaccine to test whether the positive immunological results seen in phase I trials are also present in the fight against Ebola on a large scale.
While the vaccine has the potential to bring Ebola under control, a mass rollout of an Ebola vaccine remains a distant prospect a year into the outbreak of the virus.
"It is important to remember that this vaccine is still in development and any potential future use in mass vaccination campaigns will depend on whether WHO, regulators and other stakeholders are satisfied that the vaccine candidate provides protection against Ebola without causing significant side effects and how quickly large quantities of vaccine can be made," Dr. Moncef Slaoui, chairman of global vaccines at GSK, said in a statement.
International governments, NGOs and philanthropists have donated $1 billion to fight the epidemic, but Dr. David Nabarro, the U.N. secretary general's special envoy on Ebola, told the Guardian the U.N. is looking for another $1 billion to add to the fund.
Uncommon Knowledge
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
About the writer
Before joining Newsweek, Lucy Westcott was an editorial fellow at The Wire. Previously a United Nations correspondent for the Inter ... Read more
To read how Newsweek uses AI as a newsroom tool, Click here.






