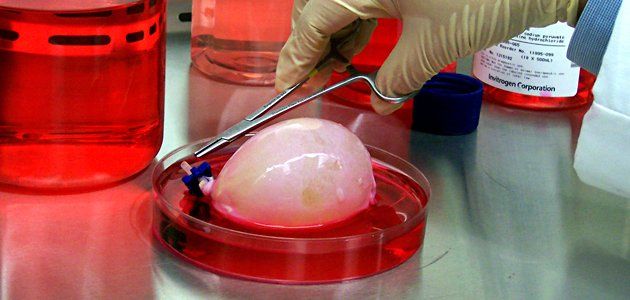
Luke Masella was born with spina bifida, a birth defect that paralyzed his bladder. By the time he was 10 years old, despite various treatments, his kidneys were failing. Toxins were building up in his blood, and he had lost 25 percent of his body weight. That's when Luke and his parents opted for a radical solution—a brand new bladder.
It might sound like science fiction, but growing new organs from scratch has already become reality. In addition to bladders, scientists have engineered new skin, bone, cartilage, corneas, windpipes, arteries, and urethras. Human organs fail for a multitude of reasons; genetic deformities, injuries, and disease can all damage them. Organ transplantation is an option, of course, but it's risky, and too often there aren't enough donated organs to meet the growing need.
So 25 years ago, a group of scientists embarked on an audacious quest: the creation of whole new organs. Brothers Joseph and Charles Vacanti at Harvard Medical School and Robert Langer of the Massachusetts Institute of Technology first promoted the idea of "tissue engineering" or "regenerative medicine." The scientists knew that every organ has a "scaffolding"—a structure that gives it shape—and many different types of cells with different functions. There are millions of cells, all arranged in an exact order.
Langer was a master at making physical structures that live within the body. He devised ways to create an organ's scaffolding using a variety of synthetic materials like biodegradable polymers and natural molecules like collagen that are part of everyone's body. Using Langer's techniques, Charles Vacanti grew cartilage in the shape of the ear of a 3-year-old child. Tissue engineers also devised an ingenious alternative approach called "decellularization": the cells of a spare organ (from an animal or human cadaver) are digested away, leaving just the natural, noncellular scaffolding.
Once a scaffolding exists, the next step in building a new organ is to drape the scaffolding with cells. Where do the new cells come from? Most often they are adult stem cells from the type of organ the doctors want to build. Sometimes adult stem cells from other organs can be chemically coaxed to turn into the cells of the new organ. For example, in pregnant women the amniotic fluid and placenta contain stem cells that can form many tissues. Surprisingly, so does fat. The unwanted fat removed by liposuction, for example, is rich in stem cells.
Ideally adult stem cells are extracted from the person who will be receiving the new organ, so that it won't be rejected. Because adult stem cells have so far worked well, scientists haven't made much use of the more controversial embryonic stem cells or the recently created induced pluripotent stem (iPS) cells that seem to behave much like their embryonic cousins.
After the scaffolding came the hard part, the part that caused most scientists outside the field to predict that growing new organs would fail. Even if you could build a scaffolding and procure the cells to drape onto that scaffolding, then what? Surely no scientist could assemble millions of cells, one by one and each in the right place, as if the organ were a giant jigsaw puzzle. It was, on its face, an insoluble problem—unless nature, somehow, helped out.
Remarkably, it has. Adult stem cells do more than turn into the types of cells needed for the new organ: they orchestrate the process of assembly. Dr. Tracy Grikscheit and her colleagues at Childrens Hospital Los Angeles saw this firsthand. Grikscheit wants to treat short bowel syndrome, a problem in some premature and full-term newborns. Without enough small intestine (bowel), the babies cannot absorb nutrients to grow and survive. Grikscheit hopes to grow the babies a new small intestine made from their own cells.
Last year, she reported an experiment with pigs. First she built a biodegradable cylindrical scaffolding. Then she opened a pig's abdomen and harvested adult stem cells from the wall of its intestine. With the belly still open, she seeded the cells onto the scaffolding. She then placed that structure in the pig's belly, onto tissue with a rich blood supply—the omentum—and sewed up the abdomen.
Seven weeks later, Grikscheit opened the pig's belly and removed the structure she had created. Under the microscope, it looked just like a small intestine: the right types of cells were in the right place. They had even attracted a blood and nerve supply. In other words, once the cells were seeded onto a familiar scaffold, they "knew" what to do. Nature had quickly assembled the jigsaw puzzle.
Next Grikscheit needs to demonstrate that the newly created bowel functions normally in pigs. Preliminary experiments suggest that it does. If follow-up work confirms the early results, it will be time to see if the approach works in the babies with short-bowel syndrome. "These kids don't need a whole new small bowel," said Dr. Grikscheit. "They just need a few inches, and they'll be able to digest everything they need. Someday I think we'll be able to make it for them."
While a tissue-engineered intestine for humans is still in the future, a handful of patients like Luke Masella are beginning to benefit from other "grown" organs. Ten years ago when Luke's bladder and kidneys were failing, the conventional treatment would have been to transform a portion of his intestine into a bladder. But that type of replacement can cause metabolic abnormalities and kidney stones, and the new "bladder" would also have a greatly increased risk of developing cancer. So tissue-engineering pioneer Anthony Atala of the Wake Forest Institute for Regenerative Medicine made Luke his own bladder. Atala created a scaffolding, harvested stem cells from Luke's original bladder, and seeded the scaffolding with those cells in the lab. Two months later, Atala attached the new bladder to Luke's original bladder, expanding it. It soon attracted its own blood supply and nerves. Now a 20-year-old sophomore at the University of Connecticut, Luke has a bladder—and kidneys—that function normally.
Even though a bladder is a relatively simple organ, Atala's achievement with Luke and others is considerable. If Grikscheit can successfully engineer and implant a more complex organ, the small intestine, it will be remarkable. How far can this go? Livers, kidneys, hearts? Perhaps, say many tissue-engineering pioneers. However, even these visionaries find it hard to imagine or to desire a tissue-engineered human brain. At its unimaginable best, it would be perfect—and empty.
Komaroff is editor in chief of the Harvard Health Letter, and a professor of medicine at Harvard Medical School.
Uncommon Knowledge
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
About the writer
To read how Newsweek uses AI as a newsroom tool, Click here.






