A new gene therapy that rescued cancer patients from the brink of death has just been approved. This week, the U.S. Food and Drug Administration (FDA) gave the green light to a cancer treatment based on immune cells that were artificially transformed into cancer-killing machines.
The newest treatment, called Yescarta, is the second such chimeric antigen receptor T-cell therapy, or CAR-T therapy, approved in the United States, and the first for adults with a certain type of blood cancer called diffuse large B-cell lymphoma.
If it seems like there's a new gene therapy announcement every few weeks, that's because there has been. In the space of two months, the FDA has also approved the first-ever CAR-T treatment, Kymriah, and signaled that a true gene therapy for a genetic form of blindness will likely be approved soon. And at the same time the FDA announced Yescarta's approval, the agency also announced yesterday that it would be rolling out a new policy to deal with similar therapies.
"We will soon release a comprehensive policy to address how we plan to support the development of cell-based regenerative medicine," FDA Commissioner Scott Gottlieb stated in a press release. "That policy will also clarify how we will apply our expedited programs to breakthrough products that use CAR-T cells and other gene therapies."

When that policy will be released and what it will actually say is still unclear, unlike the path forward for Yescarta. It will be at least a few weeks before patients can be treated with it, and only specific hospitals will be permitted to administer the therapy after going through certain trainings.
One of those hospitals will be Moffitt Cancer Center in Tampa, Florida. The center treated some of the patients in the clinical trials that supported Yescarta's approval.
"We're really excited. We treated a lot of patients on that trial, and we think that it will be a therapy that is here to stay," Dr. Frederick Locke told Newsweek. Locke was one of the principal investigators of the key clinical trial at Moffitt and has been a scientific adviser to Kite, the company that first developed Yescarta.
"These were patients who were really without hope. You know, one-in-10 chance—less than one-in-10 chance—of having a complete remission with chemotherapy," Locke said. "These are patients who have failed many kinds of therapies." But during the clinical trial, 44 percent experienced total, lasting remission. (Others saw less dramatic improvements.)
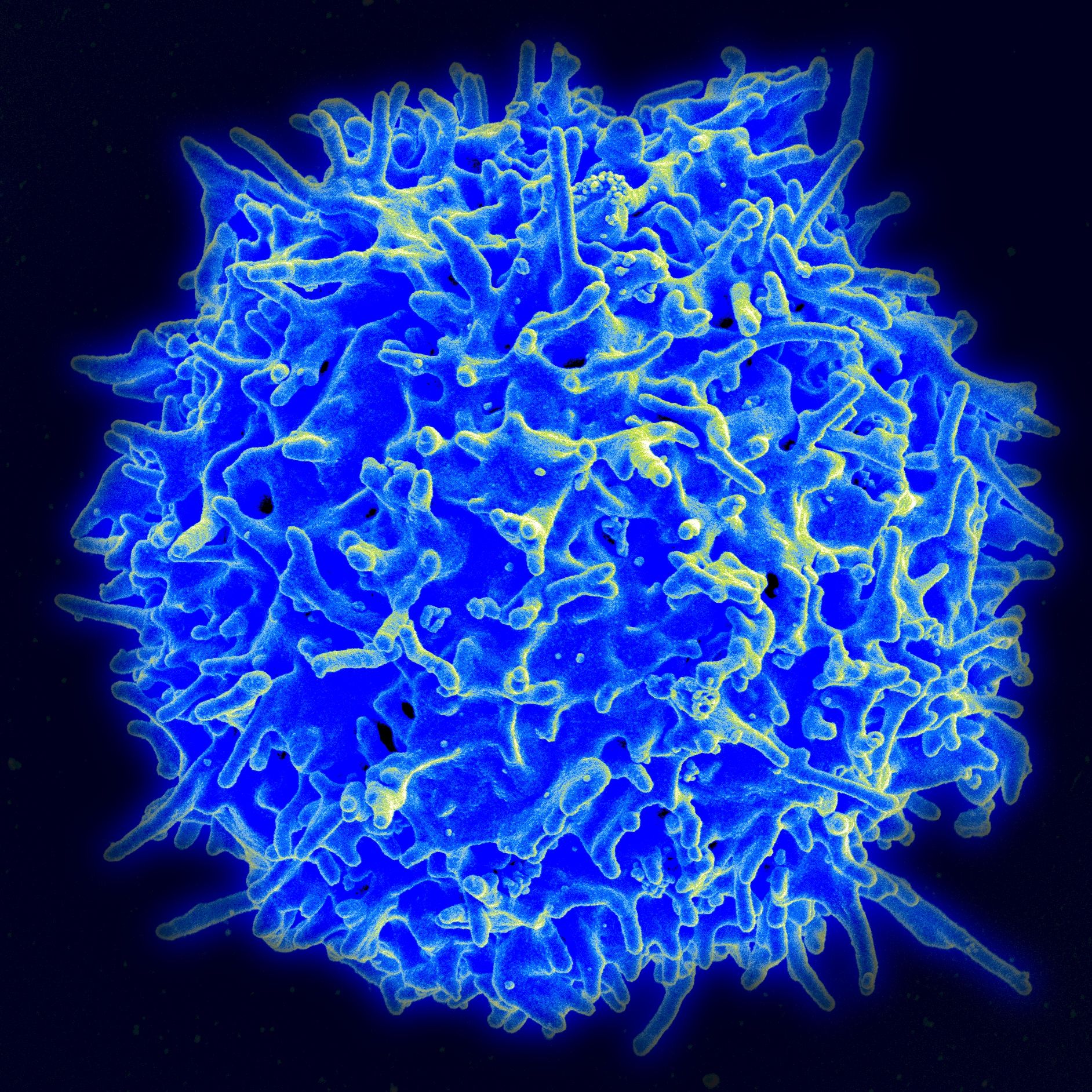
The treatment works by rejiggering a person's own immune cells and forcing them to attack blood cells that are causing the cancer. "Normally we have these T-cells in our blood and in our lymph nodes that help recognize and fight infection," Locke explained. "Some might recognize flu-infected cells, some might recognize bacteria." These T-cells can recognize out-of-place cells based on certain proteins expressed on their surface. For CAR-T, the T-cells are genetically modified to target a protein called CD-19, which is on the surface of all B-cells and only B-cells. In some lymphomas, those are the cells that are cancerous. When the modified T-cells are put back into a person's system, they look for the cells with that particular protein and attack.
But not all B-cells are cancerous, even in lymphomas; it would be like taking out a person's brain to treat brain cancer. However, unlike our brains, B-cells are not irreplaceable. "We see low B-cells after chemotherapy and in other lymphoma therapy so we can support someone," Locke said.
Theoretically, someone with brain cancer or another kind of cancer could be treated with CAR-T. But scientists would have to find a similarly specific protein for just the cancerous cells in that organ, and that's tough.
Even for people who have the kinds of cancer that can be conquered with CAR-T, the results aren't guaranteed. It's not clear entirely who benefits from the therapies, Locke said. They also come with the risk of very serious side effects. "We still need to figure out why it doesn't work for everyone. It could be the biology of the tumor, or the immune microenvironment of the tumor or the immune cells of the patient, that could suppress the CAR-T cells themselves."
And with price tags like the ones attached to these treatments, failure could be very expensive.
Kymriah costs $475,000 per dose; that does not include any of the other care that's needed around the drug to keep people alive without their healthy B-cells. Gilead and Kite announced that Yescarta would have a slightly lower price: Each dose will cost $373,000. However, when Novartis announced Kymriah's approval, the company said it would also implement a so-called "outcome-based pricing" scheme. If a patient didn't go into remission shortly after the treatment—if it didn't work, basically—then he or she wouldn't have to pay for it.
Kite representatives did not respond to messages left asking if they will be following a similar pricing scheme. But as the therapies themselves evolve in the coming years, other companies will be navigating this thorny issue—perhaps sooner rather than later.
Uncommon Knowledge
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
About the writer
Kate Sheridan is a science writer. She's previously written for STAT, Hakai Magazine, the Montreal Gazette, and other digital and ... Read more
To read how Newsweek uses AI as a newsroom tool, Click here.