Seasonal outbreaks of flu kill many thousands of Americans even in a good year, and this is a bad one.
According to the Centers for Disease Control and Prevention, each year since 2010 flu has caused "between 9.2 million and 35.6 million illnesses, between 140,000 and 710,000 hospitalizations and between 12,000 and 56,000 deaths."
This flu season, which is still inflicting misery and debility on much of the country, is ominous, and the statistics are likely to end up on the high end of those ranges.
Hospital emergency rooms and isolation wards are filling up with flu sufferers, deaths are running ahead of recent years, and except for Hawaii, the entire United States is experiencing "widespread" flu activity.
Moreover, pharmacies have reported shortages of the most common oral anti-flu medication – oseltamivir, or Tamiflu – and also of intravenous solutions needed to keep hospitalized patients hydrated.
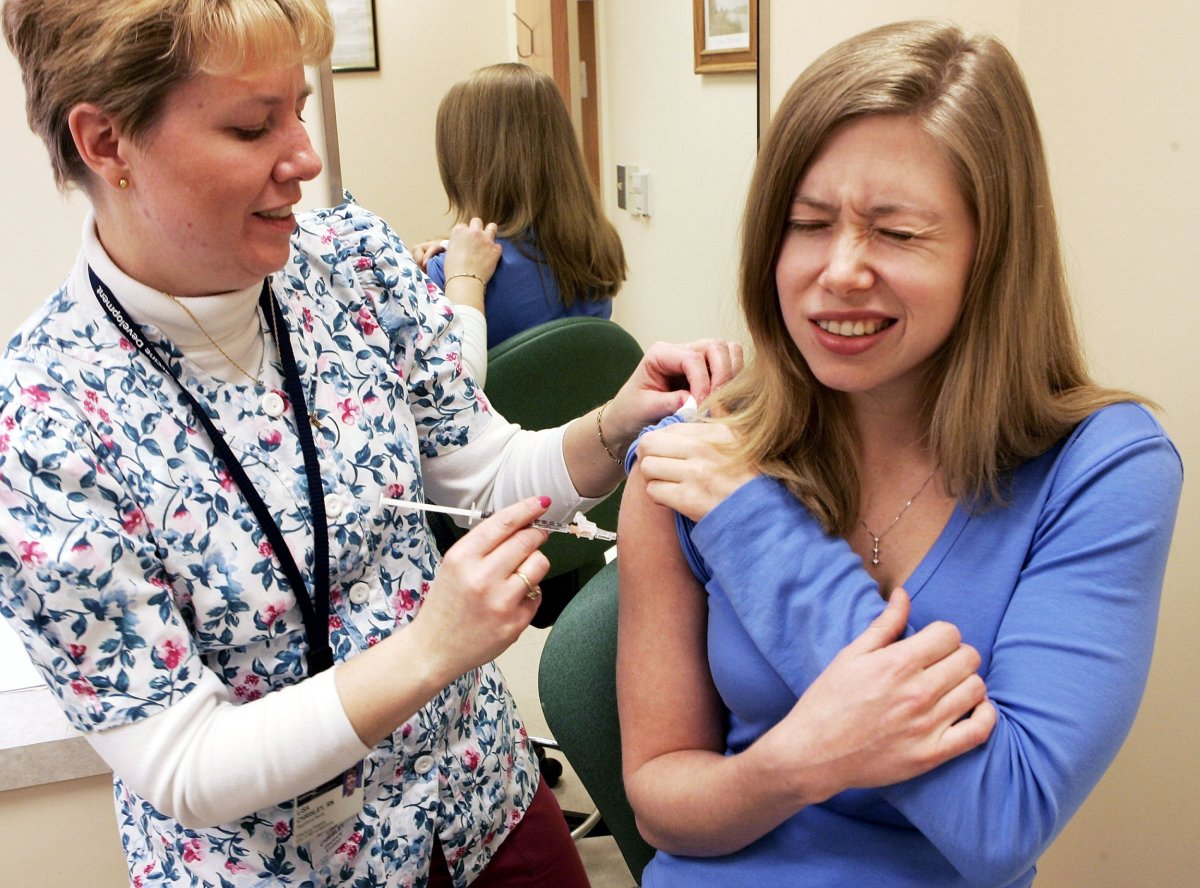
Vaccination is the key to prevention. According to estimates from the CDC, in six flu seasons, starting in 2005-06, flu vaccination against a variety of strains prevented almost 14 million cases.
That might seem impressive, but our current vaccines often are barely adequate. Too many vaccinated people still get the flu, and when it comes to research funding, our public health agencies seem little exercised about it.
Because flu viruses mutate frequently, vaccines are reformulated early in the year as a mixture of virus strains predicted to prevail during the next fall and winter. Depending on how good the selections of strains are, during a given flu season the effectiveness can vary widely.
Since the 2004-05 season, vaccines' effectivenesss has varied from 10 percent to 60 percent. This year, the vaccine is a poor match (probably around 30 percent), in part because most illnesses are caused by a virulent strain called H3N2, against which the flu vaccine typically isn't very effective.
One of the reasons for flu vaccines' relative ineffectivness is that most flu vaccine is prepared from fertilized chicken eggs, a method of production known to reduce the effectiveness against certain flu strains, particularly H3N2.
Most flu vaccines work by exposing us to non-infectious components of a virus — the viral antigens — that elicit an immune response. Regulators should encourage manufacturers to stop using chicken eggs and instead prepare vaccines in "cultured cells" — cells that have been removed from animals and grown under controlled conditions.
This method would produce vaccines with greater fidelity to the targeted flu strains. Many U.S.-licensed vaccines are already produced this way, such those for rotavirus, polio, smallpox, hepatitis, rubella and chickenpox, plus at least two flu vaccines. Regulators should require manufacturers to phase out flu vaccines produced with inferior, 70 year-old technology.
We also need more research on vaccine additives called " adjuvants," chemicals mixed with the viral antigens to further boost our immune response. But most of all, we need to accelerate research on the holy grail of flu prevention: a "universal" vaccine that would target a part or parts of the virus that remain unchanged among different strains, even during the virus's rapid mutations. A universal vaccine has the potential to provide us with long-standing protection from all strains of flu.
There is also a need for more clinical research on making flu vaccination more effective in elderly vaccinees. Within the population, a vaccine's effectiveness varies widely because it is affected by the general health and age of the recipient.
Although people 65 and older make up only 15 percent of the U.S. population, on average, they account for about 60 percent of the hospitalizations and 90 percent of the deaths attributed to seasonal flu.
Seniors respond less well to vaccines than younger people because, as we age, our immune system functions less well. Scientists at the National Institutes of Health, after reviewing 31 vaccine response studies comparing groups of different ages, called for more potent formulations for the elderly.
But exactly how strong the shot should be, and whether additional injections would boost immunity, requires more study. There is currently a flu vaccine for people over 65 that contains four times as much antigen as regular flu shots, and one that contains an adjuvant, but they have improved the shot's effectiveness only marginally.
These epidemiological and biochemical findings indicate a need to study systematically ways to increase the effectiveness of flu vaccination in the elderly.
The NIH researchers offered several suggestions for future clinical trials of vaccine efficacy:
• Stratify over-65 subjects into five- to 10-year age groups.
• Gather data on whether subjects had been vaccinated during the previous flu season.
• Ascertain whether individual subjects live in an institution (such as a nursing home) or independently.
• Measure all of the three standard blood tests that quantify the response to vaccines.
To those, I would add several more:
• Include vaccines of two types — one with virus that is inactivated and another with live but weakened virus — in separate groups in the study.
• Include vaccines with and without adjuvants (chemicals mixed with the viral antigen to boost the immune response).
• Study the effects of increased amounts of vaccine and more doses.
Ideally, another variable in such studies would be what is considered the holy grail of flu prevention: a vaccine that would target a part of the virus that remains unchanged among different strains in spite of new mutations, so that a vaccination could protect against even newly-arising strains for many flu seasons, or even permanently.
Several different approaches to a universal vaccine are being pursued, but significant challenges remain. The difficulty is that the most immunogenic part of the flu virus – protein spikes on the surface of the virion -- is the part that mutates, or drifts, from year to year, which is why vaccines need to be constantly updated. (That contrasts with the vaccines for other viral diseases such as measles and mumps, which confer long-standing immunity.)
There is a portion of the flu virion that is stable, but it is not very immunogenic, so for it to be an effective vaccine, researchers will need to find a way to make it stimulate the immune system sufficiently after it's injected.
Another approach, reported only last month [January] in the journal Science, uses genetic engineering techniques to construct a live but low-virulence virus which has been crafted in a way that when tested in mice and ferrets elicits both an antibody response and immunity mediated by a subset of white blood cells called T-cells. This dual response of antibodies and cell-based immunity is promising because T-cell-mediated immunity tends to be long-lasting.
In spite of the importance of research on universal vaccines, there is surprisingly meager federal research funding in this area. A recent New York Times article by Michael T. Osterholm, director of the University of Minnesota's Center for Infectious Disease Research and Policy, and writer Mark Olshaker tracked the government's investment in universal flu vaccine research: "The National Institutes of Health has publicly declared developing a [universal] vaccine a priority, [but] it has only about $32 million this year specifically for such research."
Another federal agency, the Biomedical Advanced Research and Development Authority, is spending $43 million on a single project in pursuit of "game-changing influenza vaccines."
These are puny efforts when compared to the roughly $1 billion spent annually on developing an HIV vaccine and the many billions that have been spent on vaccines for the Zika and Ebola viruses, which have little relevance to Americans.
An increase in research funding on adjuvants, more effective dosing regimens (especially for seniors) and better production methods are achievable changes that would better prepare us to face flu outbreaks.
In the meantime, an easy and often-neglected intervention would be for public health officials and the media to make the public more aware of the currently available anti-flu medications, Tamiflu and Relenza, that can not only often prevent the flu but also shorten the duration and severity of the illness, should it occur. The development of a universal vaccine is more challenging but it promises much greater results, and deserves more support.
This fearsome flu season should serve as a reminder: On several research and public health fronts, we need to redouble our efforts to prevent and treat the flu, especially the development of improved vaccines that prevent infections as we've done for many childhood viral illnesses.
Henry I. Miller, a physician, molecular biologist and former flu virus researcher, is the Robert Wesson Fellow in Scientific Philosophy and Public Policy at Stanford University's Hoover Institution. He was the founding director of the FDA's Office of Biotechnology.
Uncommon Knowledge
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
About the writer
To read how Newsweek uses AI as a newsroom tool, Click here.








