How did complex hydrocarbons—the chemical building blocks of life—form in space? That was the question asked by team of researchers investigating the creation of pyrene, a hydrocarbon found in meteorites.
The team successfully recreated this phenomena in a lab by simulating the hot temperature of a nearby star. Their research sheds light on the mysterious origins of the carbon in the Milky Way, and on the molecular universe itself.
Their results were published Monday in Nature Astronomy.
From carbon atoms to complex chemistry
From plants to people, life as we know it is based on carbon atoms. This element is a precursor to all known life on Earth. Hydrocarbons like pyrene are organic compounds made up entirely of carbon and hydrogen.
"Starting off from simple gases, you can generate one-dimensional and two-dimensional structures, and pyrene could lead you to 2D graphene," explained Musahid Ahmed, a scientist at Berkeley Lab, in a statement. "From there you can get to graphite, and the evolution of more complex chemistry begins."

Pyrene is part of the polycyclic aromatic hydrocarbon (PAH) family. These compounds are estimated to be responsible for some 20% of the carbon found in the Milky Way. They are made up of fused molecular rings.
Alexander M Mebel, a chemistry professor at Florida International University and one of the study authors, said: "You build them up one ring at a time, and we've been making these rings bigger and bigger."
Pyrene is formed during a heated chemical processes known as combustion.
The team investigated the chemical reactions of complex hyrocarbon 4-phenanthrenyl radical and acetylene. They had to synthesize chemical compounds in the lab that were not commercially available.
Once their gas mix was ready, the team injected it into a tiny microreactor. This device heated the gas to simulate the presence of a nearby star, before researchers used a beam of vacuum ultraviolet light to ionize the mixture.
Scientists measured how long it took for particles for form after ionization. This gave scientists a window into their parent molecules.
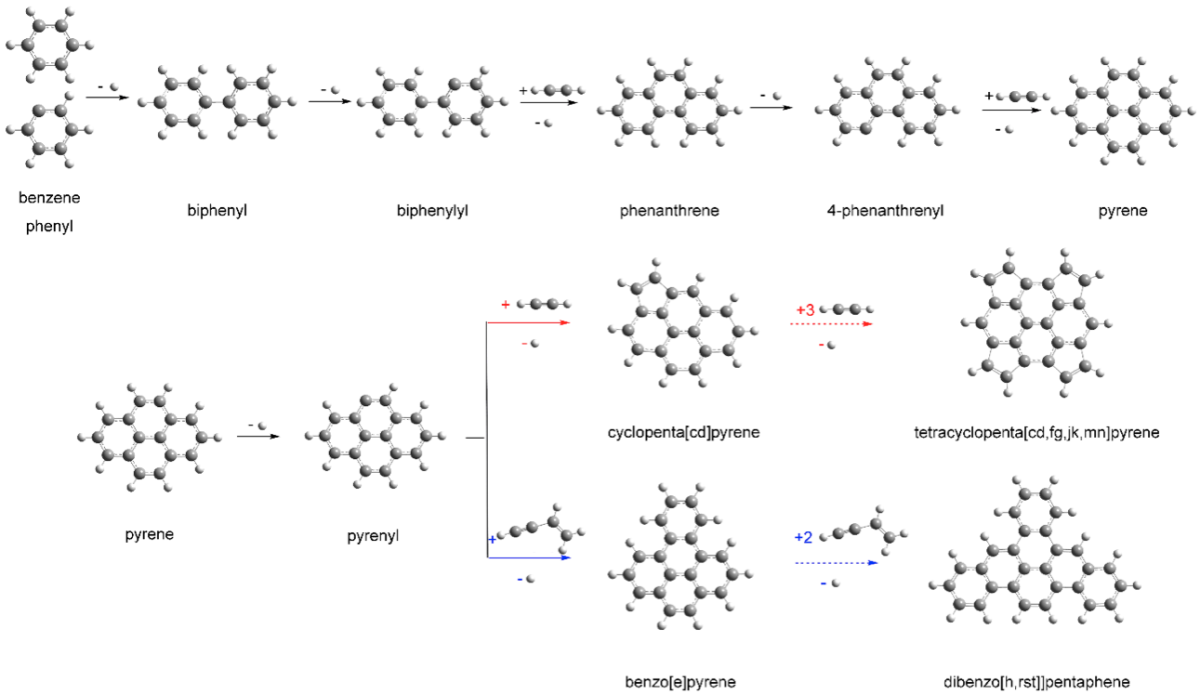
Theoretical calculations performed by Mabel described how pyrene could develop from another PAH. Combined with the practical laboratory experiment, the team used them to confirm the production of pyrene in the chemical reactions investigated.
Flames, stars and interstellar medium
Mabel's calculations can be used to study all kinds of events in space and on Earth, "from combustion flames on Earth to outflows of carbon stars and the interstellar medium," he said.
Now the team has investigated combustion, members based at the University of Hawaii will explore icy conditions and cosmic radiation to see if these can prompt the creation of hydrocarbons.
"Is this enough of a trigger?" Ahmed said. "There has to be some self-organization and self-assembly involved [to create life forms]. The big question is whether this is something that, inherently, the laws of physics do allow."
Uncommon Knowledge
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
About the writer
Katherine Hignett is a reporter based in London. She currently covers current affairs, health and science. Prior to joining Newsweek ... Read more
To read how Newsweek uses AI as a newsroom tool, Click here.








