In what has been described as a "big step forward" in the fight against malaria, scientists have caused the complete collapse of a disease-carrying population of mosquitoes by using CRISPR gene editing.
The team, led by Andrea Crisanti from Imperial College London, developed a new "gene drive," which spreads specific genes through a population over generations. The introduced gene targeted the pathways relating to sex determination—essentially, they stopped females from being produced within seven to 11 generations. Findings were published in the journal Nature Biotechnology.
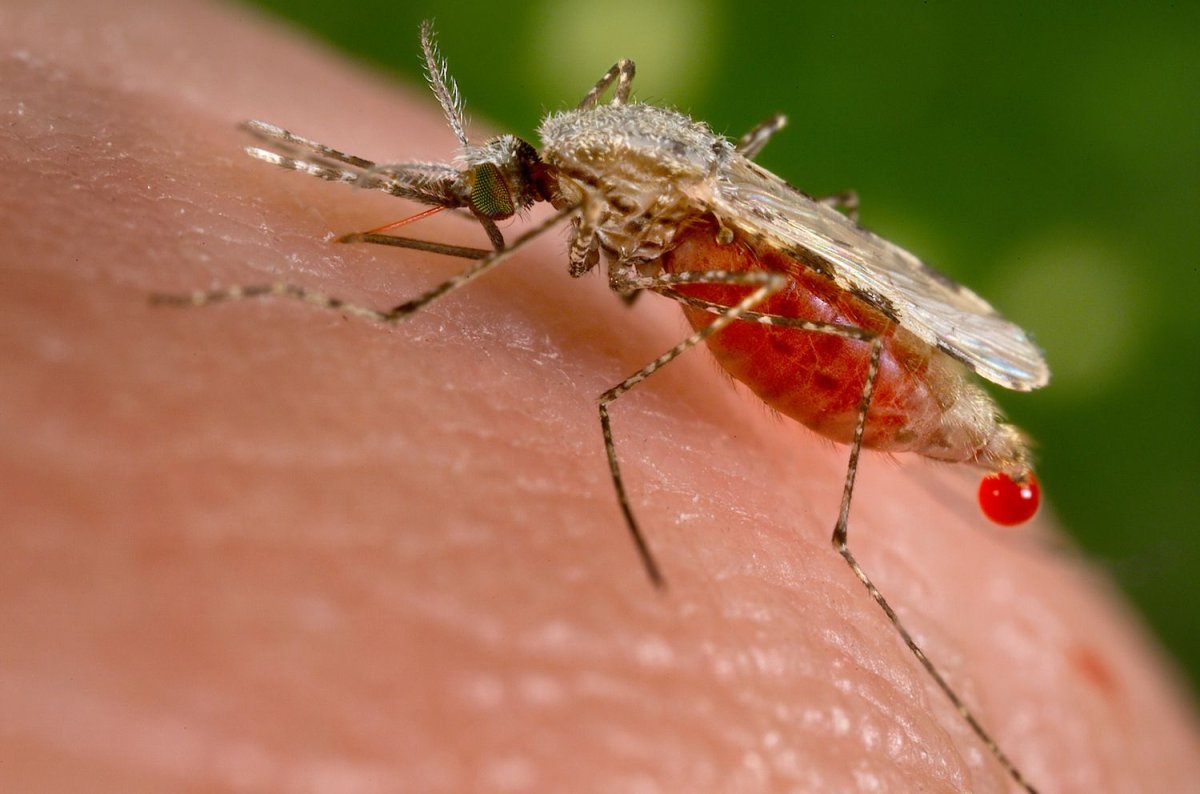
Malaria kills more than 1 million people every year, mostly children under the age of 5. Ninety percent of all cases are in sub-Saharan Africa. It is caused by the parasite plasmodium and spread via certain species of mosquito. Female mosquitoes pick up the parasite from infected people when they bite them. The parasite then reproduces and develops inside the mosquito. When the mosquito bites again, the parasite is transferred.
Finding a way to prevent the spread of malaria is extremely difficult. Experts across the globe are working on malaria vaccines but progress has been slow, and so far, those in development have had relatively low efficacy.
"It is estimated in the best-case scenario that with available technology and significant increase in funding, it will take another 30 to 40 years to eradicate malaria," Crisanti told Newsweek. "Gene drive may significantly expedite the achievement of this objective."
Genetic engineering as a means of preventing malaria spread has gained popularity in recent years. The development of CRISPR gene editing has meant scientists can remove, add or alter bits of DNA sequence. However, previous attempts to genetically alter mosquitoes have failed because the mosquitoes developed a resistance to the gene drive, and the population bounced back.
In the latest study, scientists targeted a gene called doublesex, which determines whether a mosquito becomes male or female. Researchers altered the gene so that females with two copies of the gene showed male and female traits. They failed to bite and did not lay eggs. The gene spread quickly, and after eight generations, no females were produced and the population collapsed. What's more, the mosquitoes did not develop a resistance.
The experiment was carried out in a laboratory, and the authors said that field testing would now be required to find out what happened in a more natural setting. Crisanti said it would be five to 10 years before they would consider testing this technology in the wild but that the discovery was "encouraging proof" that they were on the right track.
"Past experience has demonstrated that eliminating a few mosquito species from the environment does not cause ecological havoc," she said, adding that before any testing took place they would assess the biology and effectiveness of the gene drive under confined conditions mimicking tropical environments. They would also investigate the food webs that involve the malaria mosquito to identify prey and predators.
Fred Gould, distinguished professor of entomology at North Carolina State University, who was not involved in the study, said the results were very promising. "This is a big step forward," he told Newsweek. "There was huge excitement over using CRISPR for gene drive to fight malaria, but in the first studies the mosquitoes evolved resistance to the drive very quickly. The innovative approach used in this study suggests a way around the problem of resistance. If the drive mechanism functions under diverse environmental conditions and resistance doesn't evolve when this approach is used on a larger experimental scale, this will be a major breakthrough on the road to suppression of malaria."
Stephen Higgs, director of the Biosecurity Research Institute at Kansas State University, also welcomed the findings. "This study, although performed in a relatively small, confined-cage situation, provides a tantalizing proof of concept that suggests the use of a CRISPR-Cas9-based gene drive system could really be used to crash the population of the main malaria vector, Anopheles gambiae," he told Newsweek. "If this could be accomplished in the real world, then this approach might significantly reduce transmission of malaria parasites and therefore save many lives for years to come."
Higgs also said the results should be considered a realistic option. "These authors have a very long history and great expertise in the area, have been discussing the concept of genetic manipulation of the vector for malaria control over many years, and, with the advent of the CRISPR-Cas9 technology, are moving from concept towards application."
Uncommon Knowledge
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
About the writer
Hannah Osborne is Nesweek's Science Editor, based in London, UK. Hannah joined Newsweek in 2017 from IBTimes UK. She is ... Read more
To read how Newsweek uses AI as a newsroom tool, Click here.








