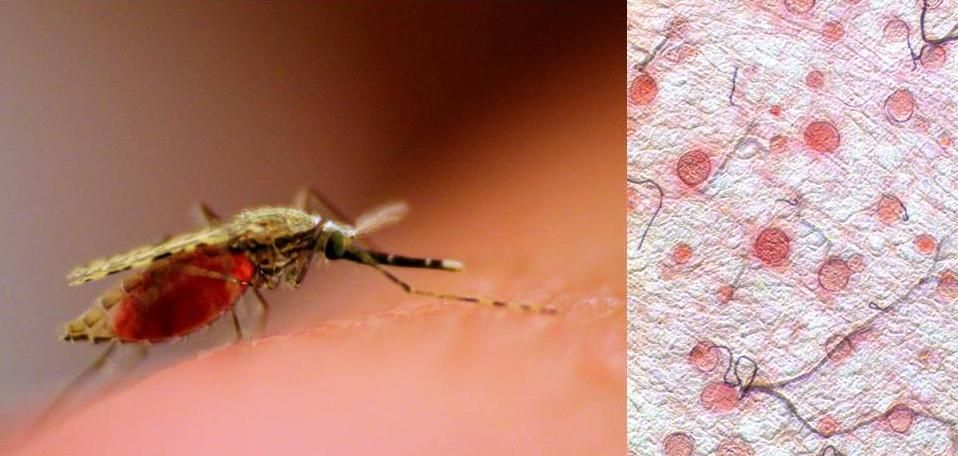
Malaria will strike more than 200 million people this year, according to the World Health Organization, and it will kill nearly half a million. Although the disease can be treated, in some parts of the world—sub-Saharan Africa, in particular—the medicines needed are often unavailable. And worse, the parasite that causes malaria is becoming more and more resistant to the drugs we have to fight it.
That's why several research groups are currently working on genetic modifications to mosquitoes that would prevent them from spreading the parasite. The latest advance comes from the University of California, Irvine, which has created a mosquito that not only doesn't transmit malaria, but that passes on this pathogen-resistant trait to 99.5 percent of its offspring.
That's quite an achievement, since many traits inherited in a more typical fashion go to only a fraction of an organism's offspring, says researcher Anthony James. His team used a gene-editing technique called CRISPR to insert two genes into the insect's genome to confer malarial resistance. Given the high rate of inheritability, the resistance would theoretically spread quickly throughout a population once it's been introduced, James says.
The UC Irvine team worked on the genome of Anopheles stephensi mosquitoes, which are a main vector of malaria in India, where there are more than 1 million cases annually and more than 500 deaths. There's reason to believe the technique would work in other species as well, according to a study describing the finding, published November 23 in the journal Proceedings of the National Academy of Sciences.
CRISPR has exploded onto the scene in the past couple years, changing the way that much genetic modification is done. It works by allowing scientists to cut DNA at a specific location in the genome and to insert desired genes. Compared with previous methods, it is much quicker and cheaper. This technique of introducing malaria resistance used by the UC Irvine team was, for example, first demonstrated in fruit flies earlier this year; just a few months later, they succeeded testing on mosquitos, says David O'Brochta, a researcher at the University of Maryland who wasn't involved in the study. His hopes are high for this kind of modification, saying that "this kind of system could help defeat malaria in the future."
The two genes James's team has introduced are modified mouse immune genes, which bind to the malaria parasites and prevent it from recognizing its host and moving around in the mosquito's body. "You can think of it as [being] blinded," James says. As a result, the parasite cannot get into the animal's salivary gland, which is where transmission takes place when mosquitoes bite humans, he says.
James says the innovation would need to be tweaked slightly before being applied in the field, and introducing genetically modified organisms into the wild would require regulatory approval from foreign countries where malaria is endemic. But he's hopeful that it could be introduced sometime in the next several years.
In the short time it has been in use, CRISPR has already raised a number of ethical questions. In April, scientists used the technique to modify human embryos; the experiment didn't result in a live birth, but did create a firestorm of controversy. James says more research needs to be done on likely long-term effects of the modification before using it in the real world. There is some concern, for example, about what might happen if the "gene drive"—the genetic mechanism that causes the trait to be passed on to nearly all offspring—were to make its way into another organism, O'Brochta says. But this seems unlikely, James says, and at this point the potential benefits of helping to stop malaria would appear to outweigh the uncertain risks.
Uncommon Knowledge
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
About the writer
Douglas Main is a journalist who lives in New York City and whose writing has appeared in the New York ... Read more





