Most metals have very high melting points, not least gold—which turns into a liquid at temperatures above 1,947 degrees Fahrenheit (1,064 degrees Celsius).
But now, researchers from Chalmers University of Technology in Sweden have found a way to melt gold at room temperature. The surprise finding came about while researchers were investigating gold samples using an electron microscope (EM).
Unlike optical microscopes that use visible light and a system of lenses to magnify small objects, EMs use electrons to produce images of extremely small objects. In fact, with this technique it is possible to study individual atoms.
In an experiment, Ludvig de Knoop, from Chalmers' Department of Physics, placed a small piece of gold in an electron microscope to see how an electric field influenced the gold atoms. He increased the electric field step-by-step while using the highest magnification.
"We wanted to see what happens to gold when it is under the influence of an extremely high electric field," de Knoop told Newsweek. "A known effect when applying such high electric fields on metals is that they evaporate, that is, they boil off from the solid metal."
When he studied the atoms in recordings taken from the microscope, he noticed something totally unexpected—the surface layers of gold had melted, despite being at room temperature.
"It wasn't until later, when we analyzed the data and the recorded movies, that we understood that we had witnessed something new and spectacular," he said. "The big surprise with our work that the outermost few atomic surface layers of gold melted before they evaporate. Further on, we realised that we could controllably switch the structure from surface melted back to being ordered by switching the electric field."
"This is an extraordinary phenomenon, and it gives us new, foundational knowledge of gold," he said in a statement.
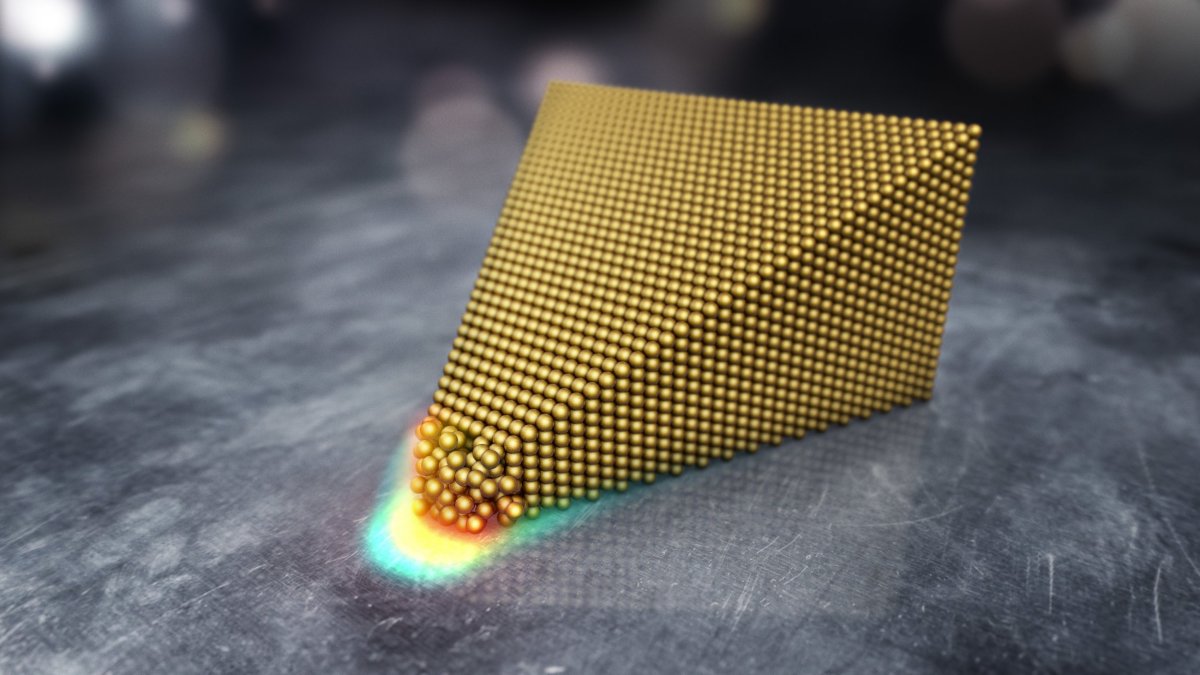
According to the researchers, the gold atoms became excited under the influence of the electric field, which caused them to suddenly lose their ordered structure, breaking the bonds between them. It was deducted that the electric field caused defects to form in the gold surface layers, melting the surface.
"We have collaborated closely with theoreticians who found from their simulations that at such high electric fields, the atoms at the surface are much more loosely bound to each other and are therefore free to move around," de Knoop said. "Important to note is that it is only the 2-3 outmost atomic layers that experience the electric field, further into the gold cone the electric field is zero and the atoms are ordered and structured in their usual way. This is an important difference compared to the melting gold by increasing the temperature."
The team also propose that the observation may be down to a phenomenon known as "low-dimensional phase transition," according to a paper describing the discovery published in the journal Physical Review Materials.
The latest results could have significant implications for the field of materials science, opening up the possibility for various applications in future, the team says.
"Primarily, the discovery is of importance for basic science," Eva Olsson, another author of the study from Chalmers, said. "Anything that provides us with new knowledge of how, in this case, a metal behaves, is interesting and significant. We could see a number of possible applications. To be able to control a few atomic layers of a metal in this fashion could for example open up for new applications in sensors, catalysts or field-effect transistors, or, for new concepts for contactless components. It is important to spread the news about the effect since it can inspire novel applications."
Uncommon Knowledge
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
About the writer
Aristos is a Newsweek science reporter with the London, U.K., bureau. He reports on science and health topics, including; animal, ... Read more
To read how Newsweek uses AI as a newsroom tool, Click here.








