
If you remember high school chemistry class at all, you might remember what happens when you drop potassium and sodium into water: It creates a big flashing, sparking explosion and remains a classic demonstration of the power of chemical reactions.
But like many seemingly simple phenomena (such as what precisely causes static electricity or why warm water can freeze faster than cold water), scientists don't know exactly understand all the details of this interaction. On the one hand, the chemistry is clear: The highly unstable pure sodium or potassium wants to lose an electron, and this splits the water atom, producing a negatively charged hydroxide ion and hydrogen and forming an explosive gas that ignites.
But, on the other hand, this reaction is so energetic that it should create a layer of steam between the metal and water that would dampen any potential fireworks, as John Timmer writes in Ars Technica. So how come it explodes?
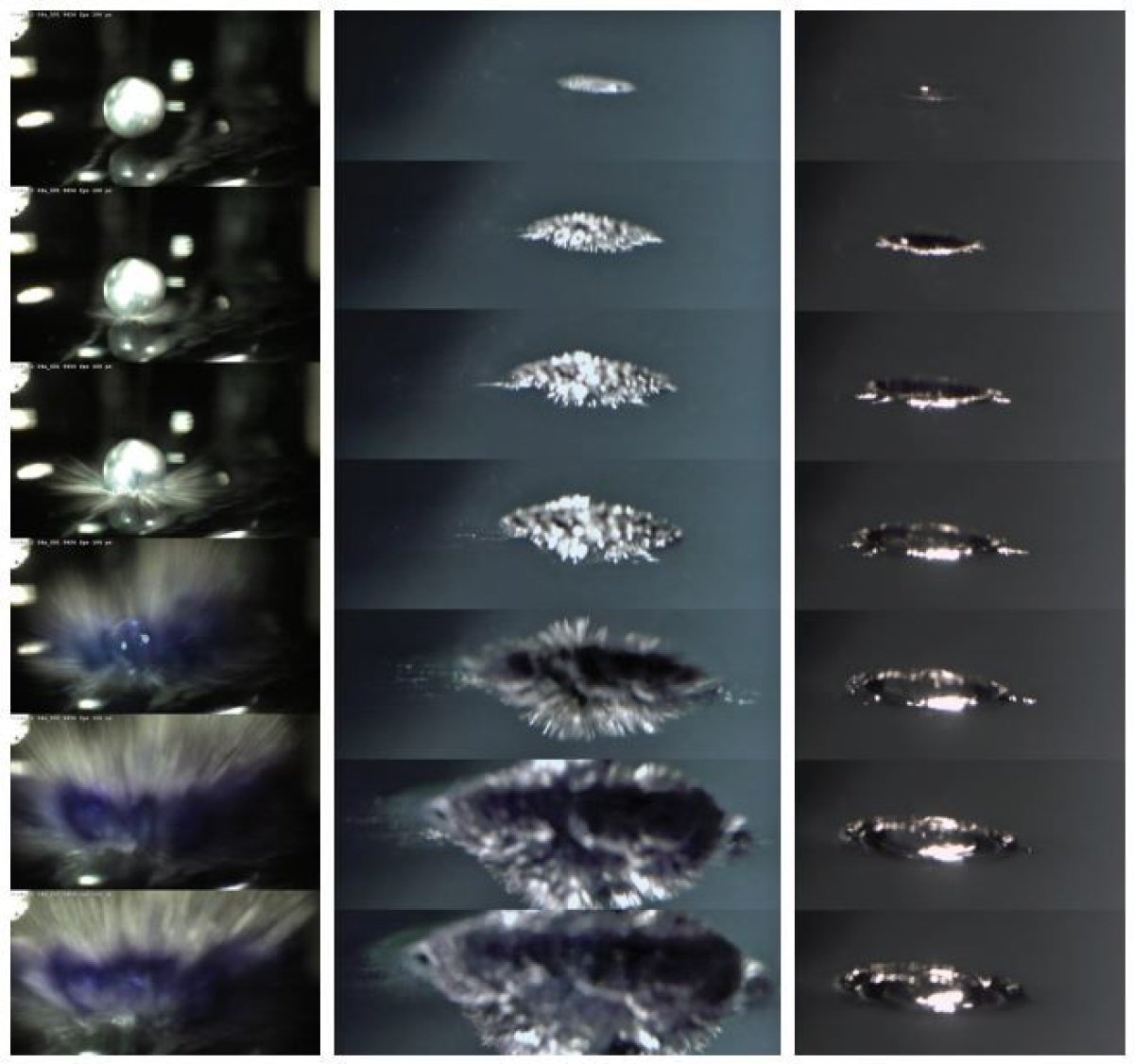
Scientists recently created an ultra-high-speed video of a drop of liquid sodium and potassium landing in water, filming from above and below to determine exactly what is going on. (They concocted this exact mixture so that it could be a uniform sphere; more complex shapes make the experiment impossible to closely observe and repeat.) Turns out, the metal shoots out into countless tiny spikes just after it hits the water, but this so-called "Coulomb explosion" happens extremely quickly, appearing visually for only less than a millisecond, at 0:47 in the video above.
Timmer more fully explains what causes the spikes:
The authors focused on what happens after the electrons leave: the metal that remains is a collection of charged ions. Computerized simulations of this reaction showed the surface of the metal rapidly forms a large positive charge, and this charge repulsion leads to a rapid expansion and disintegration of the surface. Thus, charge repulsion causes the spikes.
The authors...suggest [this process] is necessary for all of the explosive reactions between water and the elements in the first column of the periodic table. It also may explain why the explosions can be finicky, and vary depending on the state of the metal and any contaminants present.
The scientists published their explosive results last week in the journal Nature Chemistry.
Uncommon Knowledge
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
About the writer
Douglas Main is a journalist who lives in New York City and whose writing has appeared in the New York ... Read more
To read how Newsweek uses AI as a newsroom tool, Click here.






