One day, women everywhere may dissolve a postage-stamp sized piece of translucent film in their vaginas. It might look like a Listerine strip. It might be coated with compounds capable of making sperm wriggle in place, keeping them from inseminating a woman's egg. It might also halt the HIV and herpes viruses found in semen in their tracks. Oh, and those compounds might be grown in a lab inside tobacco plants.
This isn't a playful exercise in techno-futurism. This is a description of a product, about to enter clinical research phases, that is part of an emerging group of drugs that are radically changing how we treat infectious disease.
In a dark room in the basement of the biomedical research building at Boston Medical Center, Jai Marathe leans over a laser scanning microscope, adjusting a plate that holds a disk of human tissue the size of a poker chip. The flap of flesh was made from human cervical cells by a company that sells them as vaginal models to researchers. Earlier, Marathe coated it in an antibody capable of attacking the sperm cells by making them stick together, preventing them from swimming. Then she coated the tissue in semen donated by a Boston University student. The dose of antibody had been grown inside a tobacco plant at a bioprocessing lab in Kentucky. The "plantibody," as this and other antibodies grown in plants have been dubbed by the handful of companies that develop them, is the product of decades of sky-high hopes and experimentation.
When I called Richard Cone in August, he was audibly moved. Cone is a molecular biophysicist at Johns Hopkins University and was one of the earliest researchers to take an interest in plantibodies in the 1980s. A few days earlier, ZMapp, an experimental Ebola drug made from antibodies grown in tobacco plants, had been rushed to two American health workers infected with the virus. The drug, never tested on humans, appeared to save their lives. Kevin Whaley and Larry Zeitlin, the scientists behind the tiny company that developed the drug, had been postdoctoral researchers in Cone's lab in the 1990s before they went on to form Mapp Biopharmaceuticals.
Like Cone, Whaley and Zeitlin always had as a main goal the creation of a novel form of contraceptive that would also protect against disease. But, Cone says, it was hard for Mapp Biopharmaceuticals to find funding for that. "We knew that plantibodies would work. We were just following nature. But that doesn't mean that anybody will believe you," he says.
Eventually, Whaley and Zeitlin found a supporter in the U.S. Department of Defense's Defense Advanced Research Projects Agency (DARPA) arm. DARPA saw plantibodies as a potential way to make stockpiles of drugs against possible bioterror agents like Ebola, and put money toward research for a new drug to fight the disease. Then, with the current outbreak, ZMapp was suddenly thrust into the spotlight and briefly became a household name. "The story today really is a big step forward for plantibodies. This has taken so long, 30 years, to get to here," Cone says. The advent of plantibodies, he added, may be upon us. "I'm 78. I was thinking it wouldn't happen in my lifetime. But now I'm thinking it may be possible before I die."
Cone is a co-investigator on the contraceptive project, along with Mapp Biopharmaceuticals and Marathe's lab in Boston, which is run by microbiologist Deborah Anderson, another longtime plantibodies collaborator. Cone believes deeply in the potential for plantibodies to change the way medicine approaches the prevention and treatment of almost every physical disease.
"Why we have been so confident the idea will work is that we are doing just what nature has shown to do," Cone says. "It's the way mammalian mothers protect their infants." Over her lifetime, a mother is exposed to thousands of pathogens and acquires the ability to make thousands of antibodies against them. "The infant is delivered into that environment and is protected against all the same things. Breast milk is loaded with those antibodies. The first milk is basically a paste of antibodies," he says.

Passive Immunization
Today, many diseases are prevented with the use of vaccines. Vaccines work through "active immunization," by jostling the immune system into action to make its antibodies to fight off infection. Producing antibodies in a lab, and giving them to a patient as an injection or in a form that can be absorbed through the skin, is called "passive immunization." The immune system remains passive while the newly introduced antibodies ward off the pathogen on their own.
The process for making plantibodies is relatively straightforward. First, researchers settle on a human antibody capable of targeting the desired pathogen. (In the case of the plantibody contraceptive, Japanese researchers discovered the antibody in infertile women in the 1980s. In that case, the antibody attacked a coating on sperm.) Next, the researchers identify the antibody's gene, synthesize that gene and insert it into a plant virus. The virus must be capable of spreading throughout an eight- to 10-week-old plant without killing it too rapidly, and researchers have found their ideal candidate: the tobacco mosaic virus, well known due to years of tobacco industry research, because it infects the tobacco plant and kills it in two weeks.
But before that happens, as the plant grows, the virus proliferates, multiplying the antibody along with it. When the tobacco plants reach a certain level of maturity, they are harvested, and the antibody is distilled out of the plant matter. "We get a leaf that's jam-packed with monoclonal antibodies and then grind that up and use normal pharmaceutical processes to purify it," explains Charles Arntzen, a plant biologist at Arizona State University who teamed up with Mapp Biopharmaceuticals's Zeitlin to work on plantibodies in the early 2000s.
Mapp Biopharmaceuticals is one of a handful of companies experimenting with plantibodies and their myriad applications. Mapp is targeting certain types of cancer and inflammation, in addition to its work on an Ebola drug and the vaginal contraceptive. Whaley estimates there are already around 30 antibody-derived products on the market from other companies, and at least 100 more going through the research pipeline. "The thing that we like about antibodies is that they are incredibly specific," he says. They can be made to bind to something as precise as a particular part of a specific molecule on a single virus—so unlike antibiotics, which often wipe out beneficial bacteria along with the bacteria making the patient sick, antibodies tend to attack only their targets. They are also easy to make—producing more of the product simply means infecting and growing more tobacco plants.
"From a biodefense standpoint, you may not need to use the product [most of the time], but when you do, you might need to make a lot of it," says Michael Kurilla, the director of the biodefense research program at the National Institutes of Health (NIH). This feature is vital for adapting to a world where global air travel spreads infectious diseases farther faster, and where climate change could make future outbreaks worse. Governments must be equipped to respond to new strains in a flash. That's where plantibodies could come in.
"If you want to protect a large number of people immediately, you make plantibodies. They work immediately," Cone says. Most of the immunology industry is focused on making vaccines, but vaccines could take weeks to kick in. In an outbreak situation, that's time a population can't afford to lose. With antibodies, the protection is virtually instantaneous.
Plantibodies have one major downside compared with vaccines: The introduced antibodies are flushed through a person's system relatively quickly, in a matter of hours or days, and the body hasn't learned to make them itself. Vaccines teach the body to make antibodies, so one or a few doses can protect a person for years. By contrast, if a plantibody is being used to prevent a disease, the patient would need to take doses indefinitely.
Yet even if a person is vaccinated, that protection becomes useless if the pathogen mutates. In the case of certain pathogens, like HIV, that makes designing a reliable vaccine brutally difficult. "The fact is, you wouldn't [use plantibodies] if you could make a vaccine," Cone says. "But you know it has taken all this time to get a rather poor HIV vaccine, because of the way that virus works. It keeps evolving very rapidly inside that person. So you look at that and you think, How do you make a vaccine? But we're sure we could make monoclonal antibodies against it."
Drug resistance is also a worry; in an era of antibiotics overuse, virulent strains of germs are emerging that are resistant to drugs. Plantibodies fine-tuned to obliterate specific drug-resistant strains might be one answer to these superbugs. "We're looking at a lot of other applications of monoclonal antibodies for infectious diseases, both in terms of trying to address the drug resistance problem and in cases where drugs alone may not be sufficient," Kurilla says.
Whaley's lab is scrambling to produce more of the ZMapp Ebola drug while the epidemic in West Africa rages on. He says that if all goes well, the next batch of ZMapp should be ready to go into trial phases as early as January. ZMapp, Whaley says, will provide lessons on "how high of a scale we can go and how inexpensive we can go" for future products, like the plantibody contraceptive. The goal is to make them cost as little as condoms. "We figured it would take 50 or 60 acres [of tobacco] to make enough [contraceptives] for all the women in the world," Cone says.
Those Little Green Stars
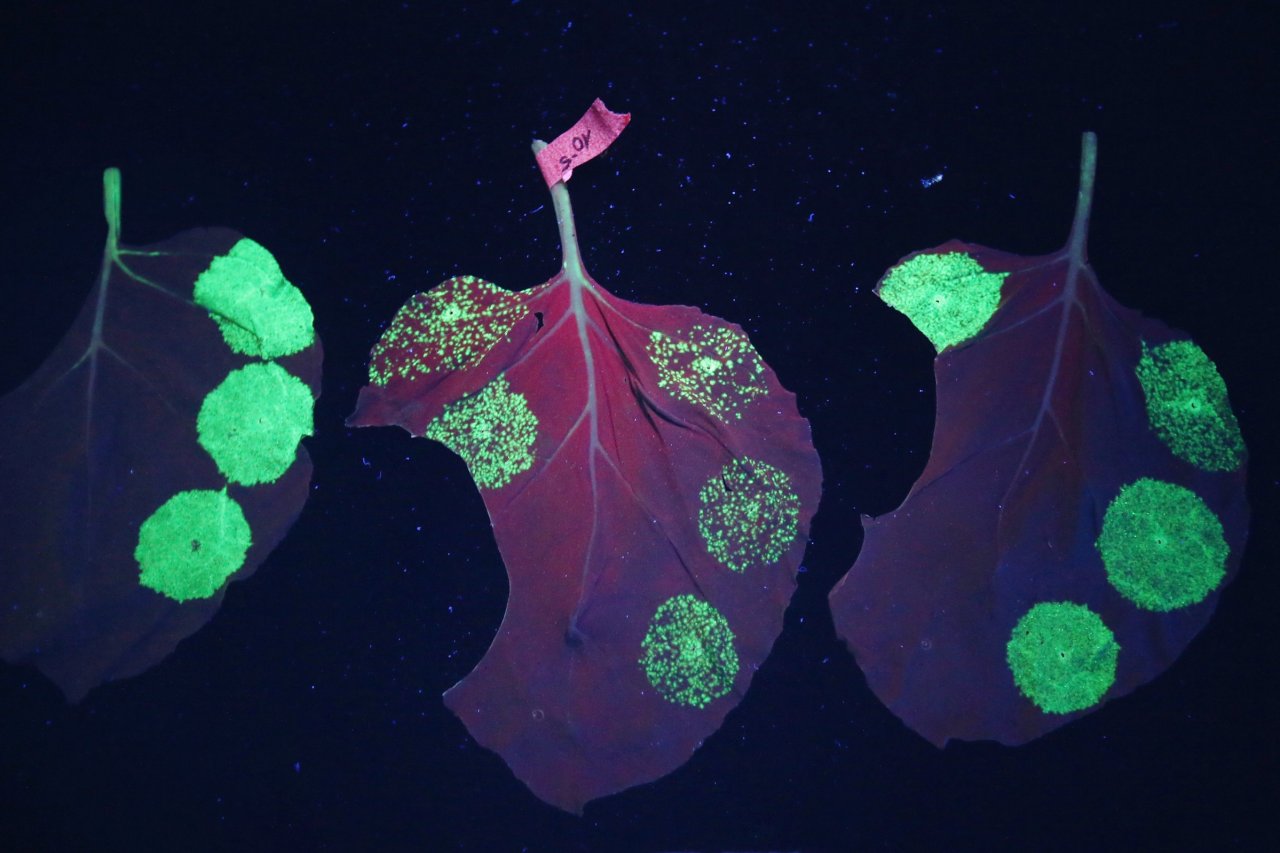
Outside, Boston was reveling in a sky-blue gem of an October day. Inside, Marathe wore a black fleece jacket to keep from shivering in the cooled basement where she is counting how many cells in the semen plasma had managed to crawl their way into the bit of cervical cell flesh.
The white blood cells in the semen, known to be transmitters for HIV, were dyed fluorescent green. Marathe let me peer into her microscope's eyepiece to look at a piece of tissue that had been treated with the plantibody, and my whole field of vision was overtaken by tiny green points of light on a dark background, like stars in the dome room of a planetarium. The top layer of the tissue was littered with them. On a monitor next to me, Marathe checked a three-dimensional graphic compiled from several images of the starry cell array. "This is good!" she says. "I have to image many more. But this is good."
On a previous, untreated sample, Marathe counted 77 green-tinted cells that had crawled their way past the flaky dead cell layer. But on the sample coated with the anti-sperm plantibody, all the cells had stayed on the surface. Not a single white blood cell—which would be carrying HIV if it had come from an HIV-positive man—had made it into the test "vagina."
Marathe's tests bode well for the future of the product, which the NIH sees as a potentially groundbreaking Multipurpose Prevention Technology, or "MPT"—a term used to describe solutions that pack sexually transmitted infection prevention into contraceptives. Right now, the only products on the market that do that are condoms. But that could soon change.
Bethany Young Holt, the executive director of CAMI Health, a research organization that works for more funding for MPT development, says there is a stigma about HIV prevention, but much less so for contraceptives. Women who don't have control over their male partner's condom will one day be able to use MPTs as if they are a standard contraceptive, while also enjoying the benefit of protecting themselves from disease.
Marathe also predicts there will be little to no systemic side effects from using the plantibody contraception—a stark contrast with birth control pills, which can have full-body side effects like changes in weight or mood. Though researchers are still trying to figure out how long the effects of each dissolvable slip of film will last, they speculate it could be anywhere from six hours to one day. If all goes well, the range of coverage—from HIV, herpes and, of course, unplanned pregnancy—will be unprecedented.
Marathe is carefully judicious about overselling the research. But her optimism peeks through for a moment. "The project can be scrapped at any step. But if this works, there will be nothing like it."
December 15, 2014: A previous version of this article incorrectly quoted Kevin Whaley as stating there are about 30 plantibody-derived products on the market. He had said there are about 30 antibody-derived products on the market.