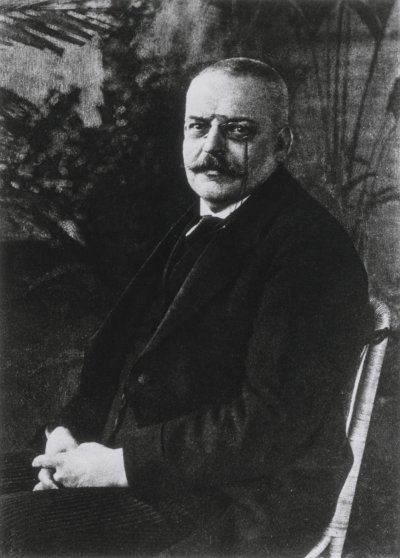Dr. Eric Reiman can't reveal the identity of the 73-year-old woman from a rugged mountain town outside Medellin, Colombia, who arrived at Boston's Logan Airport a couple of years ago for tests at Harvard Medical School. But he will say this: Finding her may well be among the most surprising developments to emerge from a nearly three-decade-long study of Colombians cursed with a gene that usually dooms its victims to full-blown Alzheimer's disease by the age of 50.
The Colombian woman is just the latest piece of evidence to emerge suggesting that the causes of Alzheimer's disease are far more complex and heterogeneous than previously understood. Despite a brain scan that revealed more amyloid-beta plaque deposition than many of her doctors had ever seen, her cognitive abilities were only mildly impaired. Which is why, even as the list of failed treatments continues to grow, many in the field have found cause for renewed optimism.
This hope is fed by an explosion in technological innovations in gene sequencing, data analysis and molecular biology, which are allowing scientists to study the progression of the disease earlier and in far more detail than previously possible. It's also fed by money: the National Institutes of Health is expected to spend $2.8 billion on Alzheimer's research in 2020—a six-fold increase since 2011, when Congress passed legislation directing the NIH to come up with an aggressive and coordinated plan to accelerate research with the ambitious goal of coming up with a way to prevent and effectively treat Alzheimer's by 2025.
That ambition reflects a growing urgency on the part of an aging public, their doctors and public health officials. By the year 2050, the number of Americans with the disease will double to 14 million, with a projected cost in treatment and care that, by some estimates, will top $2 trillion—10 percent of the present U.S. GDP. Scientists are racing to diffuse a ticking demographic time bomb. Although the field seems unlikely to meet the 2025 deadline, what researchers have learned in the past few years has given them a far more detailed and nuanced understanding of the disease. And it is raising hopes that we are finally getting closer to cracking Alzheimer's disease.
What made the Colombian woman special was not just what doctors discovered when they first scanned her brain to measure the buildup of amyloid-beta, the sticky plaques long suspected of playing a key role in the devastating cognitive decline seen in advanced Alzheimer's disease. She had the highest levels they'd ever recorded. What made the woman so special was that—despite those plaques—she seemed almost normal for her age.
"Nobody's at higher risk for Alzheimer's than she would have been," says Reiman, a neuroscientist at the Banner Alzheimer's Institute in Phoenix, who has spent the last three decades studying the loosely related, 6,000-person family cohort she belongs to in Colombia. "But she developed mild cognitive impairment about three decades after the average age in her family. And she still hasn't developed Alzheimer's dementia."
The Colombian woman's case is a potent testament to both the tantalizing promise—and the enormous frustration—that have come to characterize the pursuit of drugs to treat Alzheimer's disease. In two decades, the pharmaceutical industry has spent $600 billion in pursuit of drugs, focusing with almost single-minded intensity on compounds designed to safely reduce or prevent the buildup of deadly plaques that are one of its primary hallmarks.
Attacking plaque is precisely the point of the new Alzheimer's drug aducanumab, made by the drug maker Biogen, which was being tested in two separate clinical trials. High-ranking officials at the U.S. Food and Drug Administration have supported the drug and recently called preliminary trial results "highly persuasive." But in early November, a panel of independent experts, convened by the FDA to review data from ongoing trials, contradicted this assessment. They cited conflicting data—one trial showed a mild therapeutic effect, another trial showed none—and a lack of efficacy. "The totality of the data does not seem to provide sufficient evidence" of effectiveness, declared one of the FDA's own statisticians in a report. An FDA advisor, Dr. David Knopman of Mayo Clinic, called for a new clinical trial. "Contrary to the hope that aducanumab will help Alzheimer patients," he wrote in a report, "the evidence shows it will offer improvement to none, it will harm some of those exposed, and it will consume enormous resources."

Even if the FDA were to contravene its own experts and approve aducanumab in March, the drug appears unlikely to live up to the early promise of the class of drugs that stave off Alzheimer's by interfering with the accumulation of plaque in the brain. With aducanumab, Biogen's approach reflects the dominance of a theory called the "amyloid cascade hypothesis," which argues that amyloid-beta plaques are the first step in the condition—the kindling that eventually ignites into the fire that causes the massive cell death and memory and thinking problems that make Alzheimer's such a devastating disease. But that theory has been losing steam for years, as the case of the Colombian woman suggests.

Plaque Distraction
From the beginning, there was good reason to suspect the thick plaques that characterize the disease might also be their cause. In 1901, a 50-year-old woman named Auguste Dieter was placed under the care of Dr. Alois Alzheimer at the Frankfurt Psychiatric Hospital with an inexplicable set of symptoms, which included memory loss, disorientation, hallucinations, aphasia and delusions. "I have lost myself," she lamented shortly before her passing in 1906, according to Alzheimer's meticulous notes.
In an autopsy, Alzheimer noted the buildup of dark clumps of plaques formed by protein fragments known as amyloid-beta, along with the other two symptoms that are now considered the primary physical hallmarks of the disease that bears his name: the tangles of stringy protein molecules known as tau that clog up the space between brain cells and disrupt normal cell function, and massive cerebral atrophy caused by the death of the gray matter we rely upon to think, feel and live.
Still, the modern age of Alzheimer's research wouldn't begin until decades later, when Robert Katzman, a prominent UCSD neurologist, penned an 1978 editorial arguing that the obscure condition known as "Alzheimer disease"—a term previously reserved for those developing dementia before age 65—was actually the primary cause of what was then known only as senility. By that measure, Katzman argued, Alzheimer's disease ranked as the fourth or fifth most common cause of death in the United States and thus constituted a vastly overlooked public health challenge. In the years that followed, the first patient interest groups began to mobilize and the newly established National Institute of Aging began pouring resources into research.
Then came the discovery and study of families carrying rare mutations, like those seen in the mountains outside Medellin, Colombia, that caused them to develop the symptoms of full-blown Alzheimer's disease far earlier than elsewhere. Using the genetic tools available at the time, researchers throughout the 1990s homed in on specific mutations that appeared to be present only in family members who had developed early-onset Alzheimer's—mutations that were entirely absent in close relatives spared by the disease. Virtually all the genetic typos seemed to appear on genes that could be directly linked to the buildup of the amyloid-beta plaques in the brain.
These discoveries were among the most compelling evidence for the amyloid hypothesis, which by the early 2000s had become the dominant model used to explain how and why Alzheimer's disease progresses. And with the advent of brain scanning technologies that allowed clinicians for the first time to measure the plaques in the brains of living people, it suddenly seemed possible to track this accumulation in real time.
The implications were clear: if scientists could develop a drug capable of countering the accumulation of plaques, we could halt the progression of Alzheimer's, and the heartbreaking cognitive decline that came with it, in its tracks. "I was a student at the time—and they were heady times," recalls Scott Small, a neurologist who directs the Alzheimer's Disease Research Center at Columbia University. "We thought we had it figured it all out."
Unfortunately, things have not proven that simple. Between 1998 and 2017, there were 146 unsuccessful attempts to develop medicines to treat and potentially prevent Alzheimer's, according to a 2018 report put out by the Pharmaceutical Research and Manufacturers of America (PhRMA), the vast majority focused on the amyloid hypothesis. (The last Alzheimer's medication to receive FDA approval was Namenda in 2003, a drug that aims to temporarily boost cognitive performance by boosting the chemical messengers in the brain known as neurotransmitters).
The list of disappointing drugs that promised to cure or slow the progression of the disease is long. There was, for instance, Pfizer and Johnson & Johnson's bapineuzumab, a monoclonal antibody designed to bind to amyloid-beta. In 2012, the study's principal investor at Harvard declared human trials had produced "absolutely no evidence at all of a clinical benefit of treatment on either of the primary measures, one cognitive and one functional" in 1,100 patients with mild to moderate symptoms of the disease. Another widely anticipated drug, semagacestat, was halted after some recipients developed skin cancer and their cognition declined. In 2016, Eli Lilly & Co's solanezumab, "did nothing to improve cognition" in the phase 3, placebo-controlled trial of 2,129 patients with mild Alzheimer's disease who took the medication for more than a year.
The latest shining hope has been aducanumab, a drug whose on-again, off-again journey toward approval seems to encapsulate the infuriating ambiguity of the present moment. In 2016, the drug, developed by Biogen and Eisai, made the cover of Nature Magazine after researchers announced it had slowed cognitive decline and reduced plaques in the brains of a small group of study participants. In 2018, massive phase 3 trials kicked off in clinics around the globe, slated to finish in 2021. In March 2019, Biogen announced that a preliminary look at the results, known as a "futility analysis," had shown the medicine wasn't working as it should on the more than 3,000 hopeful early stage Alzheimer's patients participating in the study. They shut the trial down two years early and declared it a failure.
"It was an incredibly painful time for the field—painful for patients—painful for families," says Reiman, who was overseeing trials at two institutions. "[There was] concern from industry stakeholders —why are they making investments in Alzheimer's disease?—and some flight from industry. It was heartbreaking."
But that was not the end of the story. Seven months after shutting down the trial, Biogen and Esai took the unusual step of announcing they had made a mistake. The drug, they claimed, seemed to have been working after all. At a packed industry conference in December 2019, drug company representatives explained that the futility analysis had looked at only half the patients. After taking another look at the data, they found that cognitive benefits took longer than expected but had begun to kick in toward the time the trial was stopped. The company announced plans to resume an open-label trial in March and petition the FDA for approval of the drug.
The announcement was met with immense relief and cautious optimism by Reiman and his colleagues. After the conference, most agreed more data was needed to convince them the drug actually is an effective treatment. This past August, the FDA announced it had granted the drug "priority review," and will make a decision no later than March 7, 2021. If approved, it would be the first new treatment in 18 years.
Then came the November conflict. Early in the month, the FDA posted documents to its website suggesting many of the agency's clinical reviewers, including the director of the agency's neuroscience division, supported approval of the drug. The announcement came just days before the high-stakes review from an FDA advisory panel consisting of the field's leading independent experts; it caused Biogen's stock to spike by more than 40 percent. But when the panel met, committee members accused agency staff of bias and rendered a unanimous, though nonbinding, verdict: The evidence was not sufficiently convincing to recommend approval. The announcement dimmed hopes and sent Biogen's stock plummeting back to earth.
For many, this bipolar sequence of events only highlighted the folly of continuing to pursue a cure based on a single hypothesis. Some, like Columbia's Small, had already embraced the role of critic. The "basic idea" of the amyloid hypothesis, he says, "was the right hypothesis at the time. But honestly, if we were to just wake up today with all the information we have accumulated in the last 25 years, I don't think anyone would have proposed an amyloid cascade hypothesis."
"You're never going to hit the home run unless you're in the right playing field," he says. "Until now, we've been playing on the wrong playing field. We're on the right field now. The home run will be hit. I just hope it's sooner rather than later for my patients' sake."

Embracing Complexity
Many researchers agree that a promising new era in Alzheimer's research has begun—one that emphasizes the idea that a thousand flowers should be allowed to bloom in the research laboratories where scientists are seeking a cure. "We're not giving up on the approaches for amyloid but the diversity of targets we're now able to identify is creating a lot of excitement," says Richard J. Hodes, M.D.,director of the National Institute on Aging at the National Institutes of Health (NIH). "We're seeing a new wave of studies coming forward."
Hodes notes that while the NIH's National Institute on Aging is supporting 46 drug trials of interest this year, 30 of them are targets other than amyloid. It's likely just the beginning.
Back when the amyloid-beta drugs now in the clinic were being developed, he notes, just four genes had been identified that were known to be important for Alzheimer's disease. In recent years, the NIA has funded researchers collecting data from thousands of brains and has overseen a massive effort to compile them and isolate specific genetic sequences that appear to be correlated to the disease—or protection against it. In 2018 alone there were another 30 or so discovered, "which is more than there have been any previous year and the numbers are rising exponentially," says Hodes. The list of seemingly relevant genetic sequences currently tops more than 500. Research groups have narrowed this list down to more than 50 that seem promising leads for new drugs.
"The reason that's important is just as a starting point for what comes next," Hodes explains. "When we know genes that affect the risk for Alzheimer's, we can understand what those genes do, the proteins they make, the messenger RNA that comes from them. And now, with also emerging bioinformatics, we're able to put all this information together and see new molecular interactions that differ in the brains of individuals affected by Alzheimer's from those who are not."
That includes the elderly Colombian woman whose remarkable mental clarity—despite a brain full of amyloid-beta plaques—so impressed Reiman. Last winter, Reiman and his colleagues announced they had traced the cause of her unexpected mental resilience to a single "one in a million" genetic typo, one that could provide a powerful new tool to fight the disease if its effects can be replicated with a drug.
The protective genetic mutation illustrates the kind of insight that would have been impossible just a few years ago. The elderly lady was discovered during routine screening, using brain scanning technologies refined over the last decade that allow researchers to measure the amount of amyloid buildup in living brains. Reiman and his collaborators then used gene-sequencing technologies and powerful computers to compare her DNA to that of others in her kinship cohort afflicted with the disease. They quickly focused on unique changes in her genetic sequence already suspected of playing a role in brain function.
The mostly likely mutation seems to interfere with the ability of two key proteins to bind together—a binding that seems to be crucial for the progression of the deadly neural cascade that usually results in the tau tangles and cell death. Reiman and his colleagues demonstrated they could replicate this effect in the lab using small molecule drugs that consist of antibodies which interfere with the binding in a similar way. The next step is to show that it can pass the blood-brain barrier and work its magic inside actual patients.
"Out of a single case report we actually developed an antibody that might be a treatment if we can get into the brain," he says.
Drugs that replicate the mutation found in the Colombian woman could "have a more profound effect on treatment and, in particular, prevention of Alzheimer's disease." As could any number of other approaches, which presumably scientists could tailor to individual patients based on a more sophisticated understanding of how the role different genetic profiles play in how the disease develops.
"Whether amyloid has a role or not, and I continue to be agnostic, we all agree that we need a more diversified portfolio of treatments, and that there may be many different paths by which you end up developing Alzheimer's," Reiman says.


A Period of Reexamination
Gene sequencing technologies and bioinformatics are just two of the new tools that are allowing scientists to break new ground. In a laboratory perched atop a bluff overlooking the Pacific in La Jolla, California, Fred "Rusty" Gage, President of the Salk Institute, is transforming skin cells taken from Alzheimer's patients into stem cells—undifferentiated or partially differentiated cells that can be transformed into specific types of cells. In this case, Gage is turning the stem cells into baby neurons in petri dishes. The next step is to meticulously track their degeneration as they mature, in the hope of understanding precisely what goes wrong when brain cells prone to Alzheimer's age and how mutations unique to individual patients can disrupt normal cell function. Gage was struck by the vast variety of different ways he witnessed neurons derived from different patients begin to break down.
Through this work, Gage has come to believe that Alzheimer's is not simply one disease, but many—each caused by the collapse of one or more of the myriad cellular systems crucial to upkeep and health of the neurons that do our thinking. Genetic flaws may hasten the collapse of these cellular systems, but the most powerful force bringing each one about is something far more universal and inescapable: the relentless passage of time.
"We are going through a period of reexamination of our underlying principles regarding Alzheimer's disease," he says. "The biggest risk for Alzheimer's disease is age, and we don't really understand aging very well. You're not going to get Alzheimer's disease at 11. So there's a lot of interest right now in setting up models where you can look at the disease through these mutations, for example, but you have to add aging in there and understand aging."
Generally, there are eight different kinds of cellular processing that seem to go wrong in cells as we age—any one of which, Gage argues, could potentially catalyze the systemic collapse that occurs in the brains of those with Alzheimer's.
As we age, the power plants of the cell, known as mitochondria, lose the ability to effectively process the fuel needed to power cellular processes. The cell's garbage collection services begin to slow down, which causes zombie cells, misfolded proteins and other cellular debris to accumulate in the cell. Meanwhile, the quality control team of the cell—enzymes that detect mistakes in DNA and fix them—stop working, creating more potential for chaos. The cells become unhealthy and secrete signals that cause inflammation. The DNA begins to deteriorate, and the on-off switch for certain genes gets broken off.
"All of these different events accumulate with age," Gage says. "And what's happening right now that I find very exciting is that we are beginning to understand how interrelated all these problems are. All these systems need to be working, and you could have perturbation in one of them and that will affect other ones."
Alzheimer's, under Gage's vision, is not a disease where brain cells suddenly die, as if struck down instantaneously by a heart attack. Rather, the cells seem to suffocate to death on cellular garbage, or collapse because the walls have fallen in, or short out because energy production has somehow gone awry. Gage's petri dishes are allowing him to dysregulate different systems and see how different populations of Alzheimer's-induced cells respond to different drugs, based on which systems have been perturbed.
It's entirely possible, says Gage, that drugs designed to reduce amyloid-beta may work on some patients—but not on others. And despite the failures and disappointments of recent decades, some researchers argue the progress is not as discouraging as it may appear.
Indeed, plenty of researchers still believe that amyloid-beta may hold the key to understanding the disease. In recent years, many have begun to suggest that perhaps the reason drugs that target amyloid-beta have so far failed to cure the disease is not that the harmful plaques aren't essential to the disease. They may have failed because the drugs are being administered to the patients too late.
Still, even those researchers who are still primarily focused on plaques have recently developed a newfound appreciation for the disease's heterogeneity and complexity. "Alzheimer's is a very complex set of changes in the brain," says Dr. Reisa Sperling, a neurologist who directs the Center for Alzheimer's Research and Treatment at Boston's Brigham and Women's Hospital. She is leading several of the efforts aimed at testing out the effectiveness of some of the amyloid-beta drugs on patients who are at a relatively early stage in the disease
The failure of any system involved in protein handling can have profound consequences. "I think that is what goes wrong in all neurodegenerative diseases, not just Alzheimer's disease—that these proteins you make normally you can't figure out how to get rid of them as you get older," she says. "The system breaks down. I totally agree with that. It just happens to be that the two proteins we have the most problem handling in Alzheimer's disease are amyloid and tau."
In the early 1970s, Nixon declared war on cancer and the National Heart Lung and Blood Institute was going gangbusters for heart disease in America. At the time, however, Alzheimer's disease was not recognized as a disease of older adults. "When I look at the history of Alzheimer's disease, I see a patchwork of progress and failure," says Jason Karlawish, a Professor of Medicine, Medical Ethics and Health Policy, and Neurology at Penn, who cares for patients at the Penn Memory Center. "There's been a lot of decent progress around understanding what is the disease, how to diagnose it, and therefore get closer to ideas about what might be plausible, druggable targets. For a disease that wasn't even recognized until 1976, that's pretty darn good progress. That's why there's reason to be optimistic."
How long it will take to gain the upper hand remains the big question. But with scores of new trials moving toward maturity and a flood of federal money, researchers expect to make strides in the decades ahead. Most important, many scientists believe they are finally on the right track.