Scientists may be one step closer to answering the age-old question of where life came from.
The earliest records of life on Earth date back to around 3.7 billion years ago, according to the Smithsonian National Museum of Natural History. However, scientists believe that the chemical conditions required to support life likely existed around 4 billion years ago.
To carry out all of the important chemical reactions that allow life to exist, the earliest life forms needed some way of separating this internal chemistry from the outside world. Enter protocells: spherical collections of fats that create a protective bubble around the cell's contents.
These fat bubbles eventually evolved into the complex membranes that surround our cells today. But where did they come from?
In a new study published in the journal Chem, scientists from Scripps Research outlined a plausible pathway for how these protocells may have first formed and chemically progressed to allow them to diversify their function.
The focus of their discovery was a family of molecules called phosphates—molecules containing phosphorus and oxygen, which are present in nearly every chemical reaction in the body. Our cell membranes are made up of molecules called phospholipids, which consist of a phosphate "head" and a fatty tail and self-assemble into a protective bubble around our cells.
"We've now discovered a plausible way that phosphates could have been incorporated into cell-like structures earlier than previously thought, which lays the building blocks for life," Ramanarayanan Krishnamurthy, co-corresponding senior author and professor in the Department of Chemistry at Scripps Research, said in a statement. "This finding helps us better understand the chemical environments of early Earth so we can uncover the origins of life and how life can evolve on early Earth."
In their study, Krishnamurthy, co-corresponding senior author Ashok Deniz, and their teams at Scripps Research identified three likely mixtures of chemicals that may have come together to form these early protocells. These included fatty acids and glycerol, the molecules that hold the phosphate heads and fatty tails together in our cell membranes.
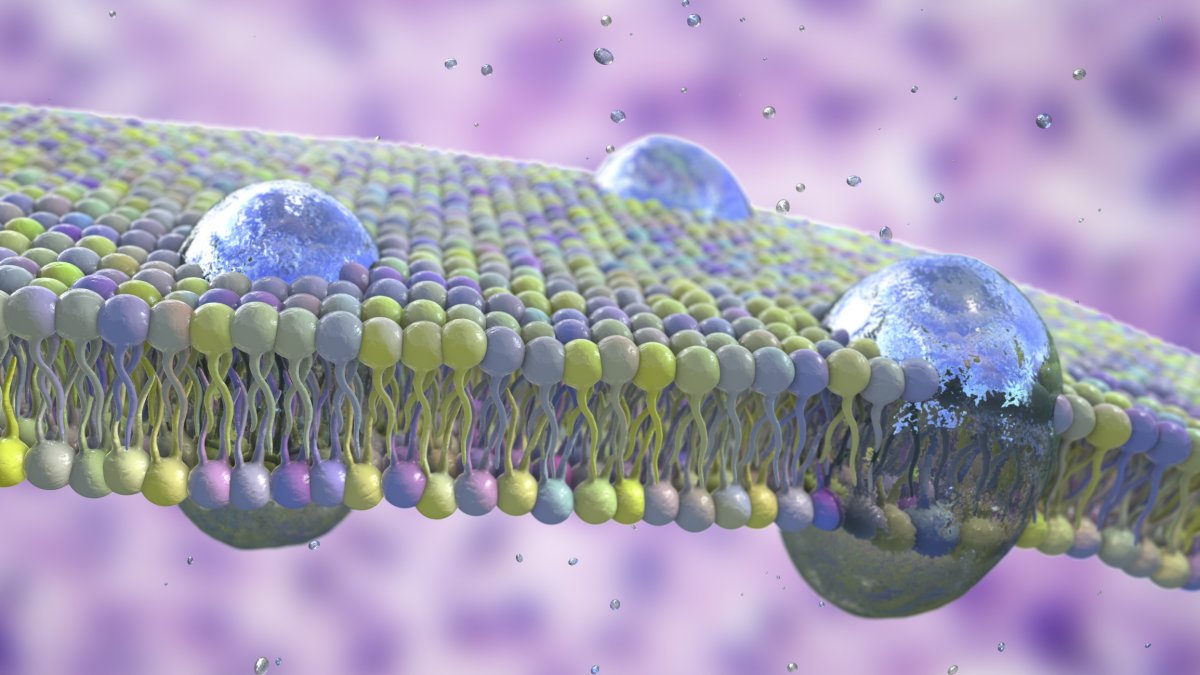
The team then varied the conditions and composition of these mixtures before testing whether any protocell-like bubbles spontaneously assembled. At the end of the experiment, the team found that phosphates had been incorporated into the structure of the protocell-like bubbles.
"We've discovered one plausible pathway for how phospholipids could have emerged during this chemical evolutionary process," Deniz said in a statement. "It's exciting to uncover how early chemistries may have transitioned to allow for life on Earth. Our findings also hint at a wealth of intriguing physics that may have played key functional roles along the way to modern cells."
In future work, the team plans to study how these protocell-like bubbles can fuse and divide to better understand the dynamic processes of protocells in this early environment.
Uncommon Knowledge
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
About the writer
Pandora Dewan is a Senior Science Reporter at Newsweek based in London, UK. Her focus is reporting on science, health ... Read more
To read how Newsweek uses AI as a newsroom tool, Click here.






